EU pesticide approvals, renewals, and extensions in 2023
- Pesticides
Summary
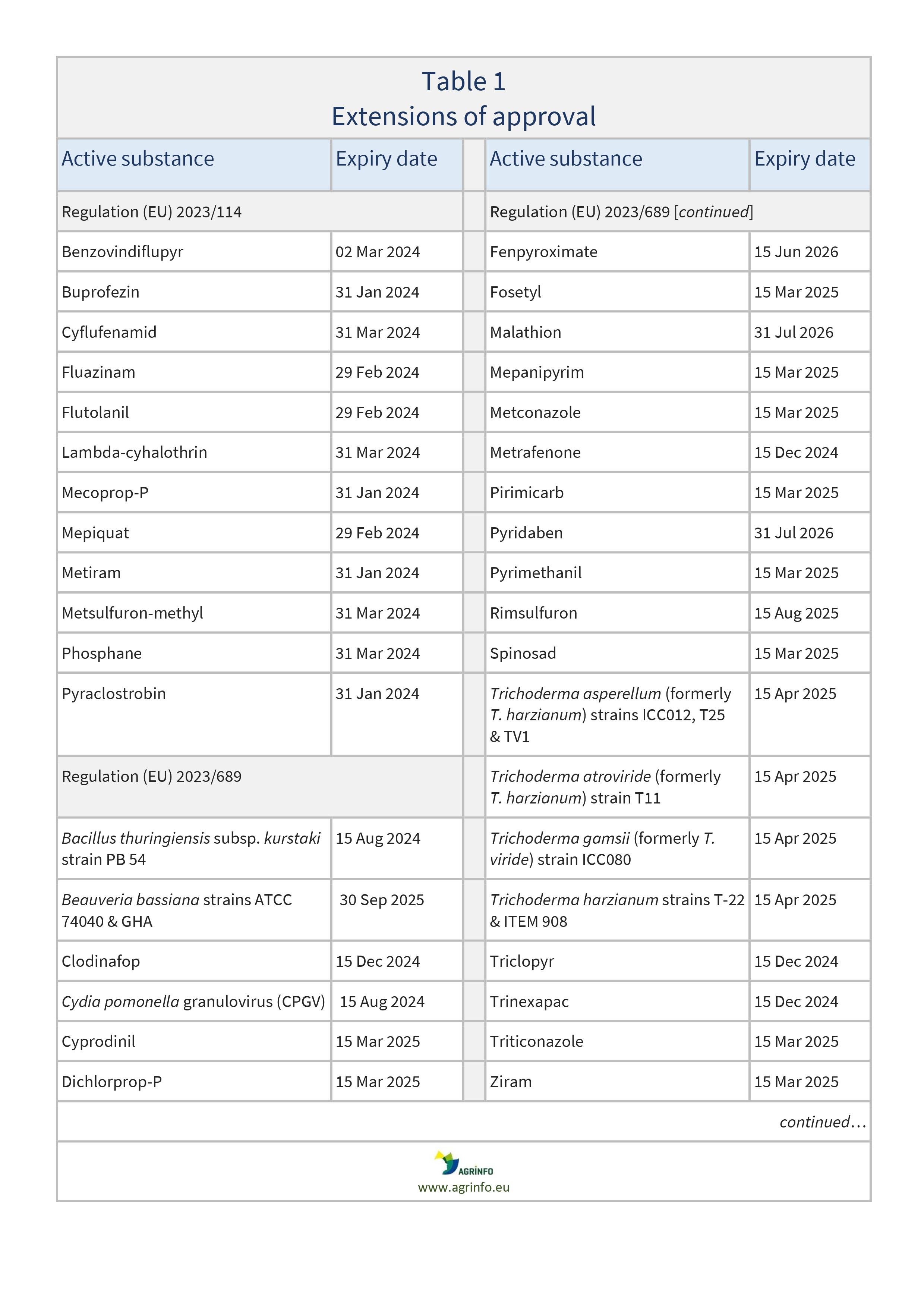
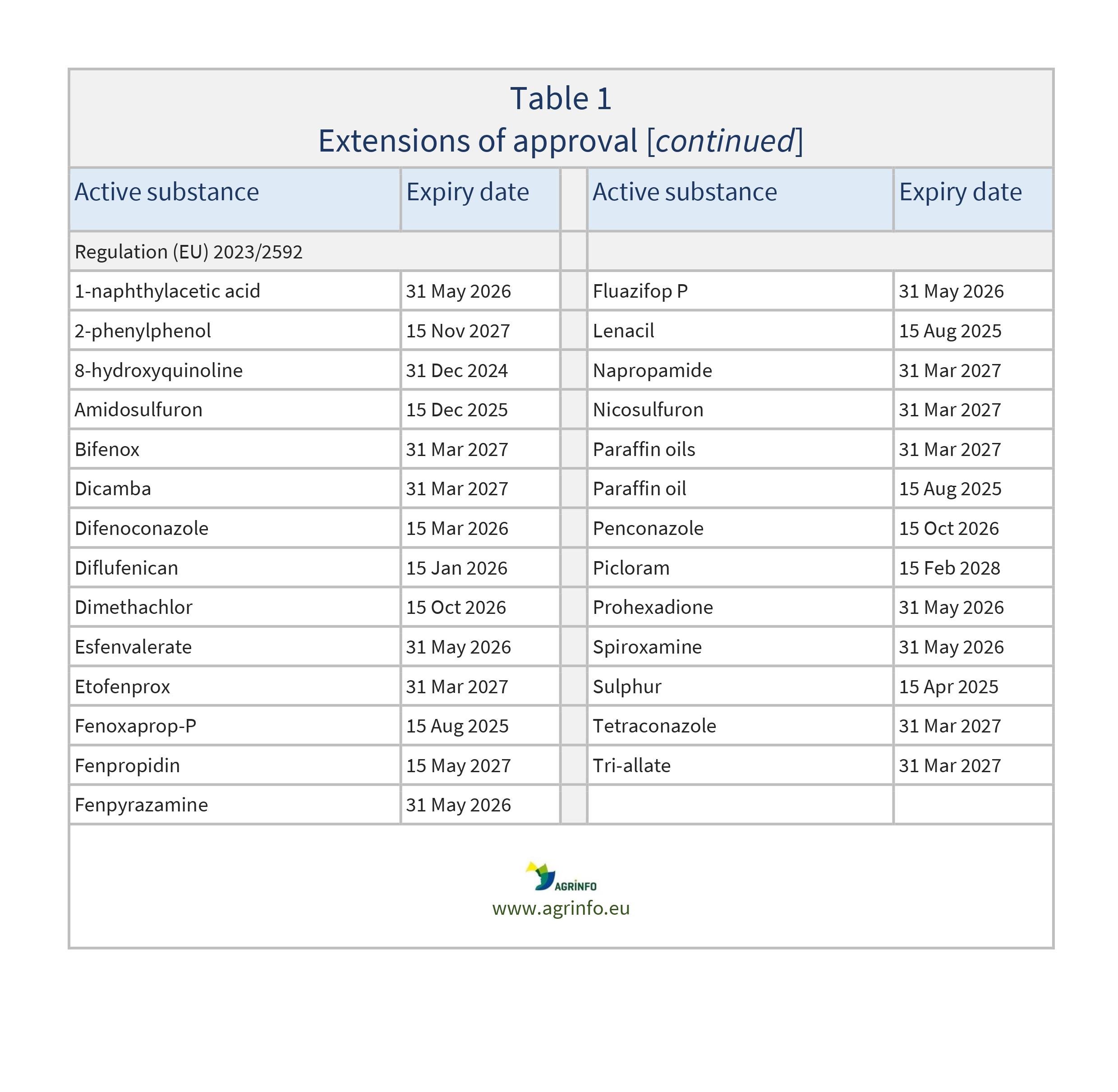
In 2023, the EU extended its approval for use of the active substances set out in Table 1. Most recently, approvals were extended for 1-naphthylacetamide, 1-naphthylacetic acid, 2-phenylphenol (including its salts, such as sodium salt), 8-hydroxyquinoline, amidosulfuron, bifenox, dicamba, difenoconazole, diflufenican, dimethachlor, esfenvalerate, etofenprox, fenoxaprop-P, fenpropidin, fenpyrazamine, fluazifop P, lenacil, napropamide, nicosulfuron, paraffin oils and paraffin oil [in separate approvals], penconazole, picloram, prohexadione, spiroxamine, sulphur, tetraconazole, and tri-allate.
Extensions, approvals, and renewals of pesticides for use in the EU introduced in 2023
Commission Implementing Regulations (EU) 2023/114, 2023/199, 2023/223, 2023/200, 2023/689, 2023/918, 2023/998, 2023/999, 2023/1000, 2023/1001, 2023/1002, 2023/1003, 2023/1004, 2023/1005, 2023/1446, 2023/1447, 2023/1488, 2023/1755, 2023/1756, 2023/1757, 2023/2589, 2023/2591, 2023/2592
Update
In 2023, the EU extended its approval for use of the active substances set out in Table 1. Most recently, approvals were extended for 1-naphthylacetamide, 1-naphthylacetic acid, 2-phenylphenol (including its salts, such as sodium salt), 8-hydroxyquinoline, amidosulfuron, bifenox, dicamba, difenoconazole, diflufenican, dimethachlor, esfenvalerate, etofenprox, fenoxaprop-P, fenpropidin, fenpyrazamine, fluazifop P, lenacil, napropamide, nicosulfuron, paraffin oils and paraffin oil [in separate approvals], penconazole, picloram, prohexadione, spiroxamine, sulphur, tetraconazole, and tri-allate.
What is changing?
In 2023, the EU extended its approval of the substances set out in Table 1.
The EU also:
- approved Trichoderma atroviride AT10 as a low-risk active substance (expiry 20 Feb 2038)
- renewed Pseudomonas chlororaphis strain MA 342 only as fungicide for seed dressing in closed seed dressing machinery (expiry 28 Feb 2038)
- renewed the approvals of Bacillus amyloliquefaciens strain QST713; Bacillus thuringiensis subsp. aizawai strains ABTS-1857 & GC-91; Bacillus thuringiensis subsp. israeliensis (serotype H-14) strain AM65-52; Bacillus thuringiensis subsp. kurstaki strains ABTS 351, SA11, SA12 & EG2348; aluminium ammonium sulphate, and ethephon
- renewed the approval of quartz sand, Cydia pomonella granulovirus (CpGV), and fat distillation residues as low-risk active substances
- decided not to approve Citrus lemon essential oil as a basic substance.
Why?
Non-approval of lemon essential oil
EFSA (2021) concluded that the main hazards associated with lemon essential oil are its toxicity by inhalation and skin irritation. Lemon essential oil also presents significant risks to the environment: D-lemonene, its main component, is very toxic to aquatic organisms, with long-lasting effects. The European Commission concluded that this substance cannot be approved.
Timeline
A comprehensive list of active substances, their legal status, and expiry dates can be found on the Commission’s Active substances, safeners and synergists database.
Recommended Actions
Suppliers to the EU market should monitor developments in EU approvals in order to help develop longer-term strategies on agricultural practices that are in conformity with EU rules.
Background
Approvals
A plant protection product or pesticide contains one or several active substances. Active substances must meet the established approval criteria (Regulation (EC) No 1107/2009, Art. 4), which include no harmful effects on human health or the environment.
Depending on their characteristics, some active substances can be approved as low risk substances that fulfil certain criteria (Regulation (EC) 1107/2009, Annex II(5)). Low risk substances are approved for 15 years instead of the standard 10 years, and enjoy more favourable data protection (13 years). Authorisation procedures are generally also shorter.
Approval renewals
The Commission has established a series of work programmes to manage the renewal process. The fifth work programme covers substances with approval expiring between 1 January 2022 and 31 December 2024. Each substance is allocated to a Member State for evaluation.
If no application is received for extension of approval, the initial expiry date will remain.
Extension of approvals
If an application for renewal cannot be fully evaluated before the expiry date, the approval can be extended to allow the evaluation to be completed.
Resources
Online resources from the European Commission:
EFSA (2021) Outcome of the consultation with Member States and EFSA on the basic substance application for approval of lemon essential oil to be used in plant protection as an acaricide, insecticide and fungicide in fruit trees (citrus). EFSA Supporting Publication, EN-6873.
Sources
Regulation (EU) 2023/114 on the extension of the approval periods of the active substances benzovindiflupyr, buprofezin, cyflufenamid, fluazinam, flutolanil, lambda-cyhalothrin, mecoprop-P, mepiquat, metiram, metsulfuron-methyl, phosphane and pyraclostrobin
Regulation (EU) 2023/199 approving the low-risk active substance Trichoderma atroviride AT10
Regulation (EU) 2023/223 renewing the approval of the active substance Pseudomonas chlororaphis strain MA 342
Regulation (EU) 2023/200 on the non-approval of lemon essential oil (Citrus limon essential oil) as a basic substance
Regulation (EU) 2023/689 as regards the extension of the approval periods of the active substances Bacillus subtilis (Cohn 1872) strain QST 713, Bacillus thuringiensis subsp. aizawai strains ABTS-1857 and GC-91, Bacillus thuringiensis subsp. israeliensis (serotype H-14) strain AM65-52, Bacillus thuringiensis subsp. kurstaki strains ABTS 351, PB 54, SA 11, SA12 and EG 2348, Beauveria bassiana strains ATCC 74040 and GHA, clodinafop, Cydia pomonella granulovirus (CpGV), cyprodinil, dichlorprop-P, fenpyroximate, fosetyl, malathion, mepanipyrim, metconazole, metrafenone, pirimicarb, pyridaben, pyrimethanil, rimsulfuron, spinosad, Trichoderma asperellum (formerly T. harzianum) strains ICC012, T25 and TV1, Trichoderma atroviride (formerly T. harzianum) strain T11, Trichoderma gamsii (formerly T. viride) strain ICC080, Trichoderma harzianum strains T-22 and ITEM 908, triclopyr, trinexapac, triticonazole and ziram
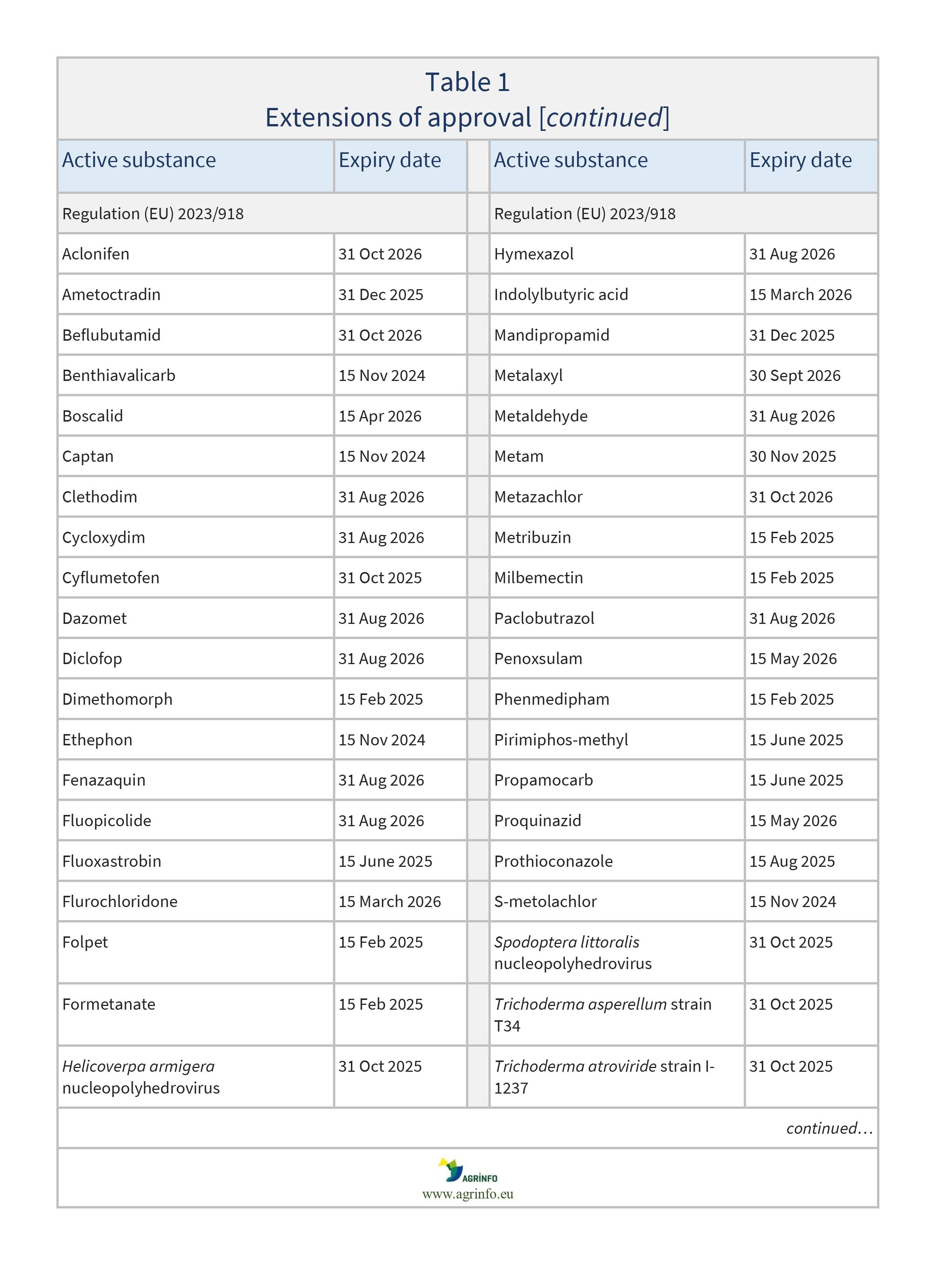
Regulation (EU) 2023/918 as regards the extension of the approval periods of the active substances aclonifen, ametoctradin, beflubutamid, benthiavalicarb, boscalid, captan, clethodim, cycloxydim, cyflumetofen, dazomet, diclofop, dimethomorph, ethephon, fenazaquin, fluopicolide, fluoxastrobin, flurochloridone, folpet, formetanate, Helicoverpa armigera nucleopolyhedrovirus, hymexazol, indolylbutyric acid, mandipropamid, metalaxyl, metaldehyde, metam, metazachlor, metribuzin, milbemectin, paclobutrazol, penoxsulam, phenmedipham, pirimiphos-methyl, propamocarb, proquinazid, prothioconazole, S-metolachlor, Spodoptera littoralis nucleopolyhedrovirus, Trichoderma asperellum strain T34 and Trichoderma atroviride strain I-1237
Regulation (EU) 2023/998 renewing the approval of the active substance Bacillus thuringiensis subsp. kurstaki ABTS-351
Regulation (EU) 2023/999 renewing the approval of the active substance Bacillus thuringiensis subsp. israelensis strain AM65-52
Regulation (EU) 2023/1000 renewing the approval of the active substance Bacillus thuringiensis subsp. aizawai GC-91
Regulation (EU) 2023/1001 renewing the approval of the active substance Bacillus amyloliquefaciens strain QST 713
Regulation (EU) 2023/1002 renewing the approval of the active substance Bacillus thuringiensis subsp. aizawai strain ABTS-1857
Regulation (EU) 2023/1003 renewing the approval of the active substance Bacillus thuringiensis subsp. kurstaki EG2348
Regulation (EU) 2023/1004 renewing the approval of the active substance Bacillus thuringiensis subsp. kurstaki SA-11
Regulation (EU) 2023/1005 renewing the approval of the active substance Bacillus thuringiensis subsp. kurstaki SA-12
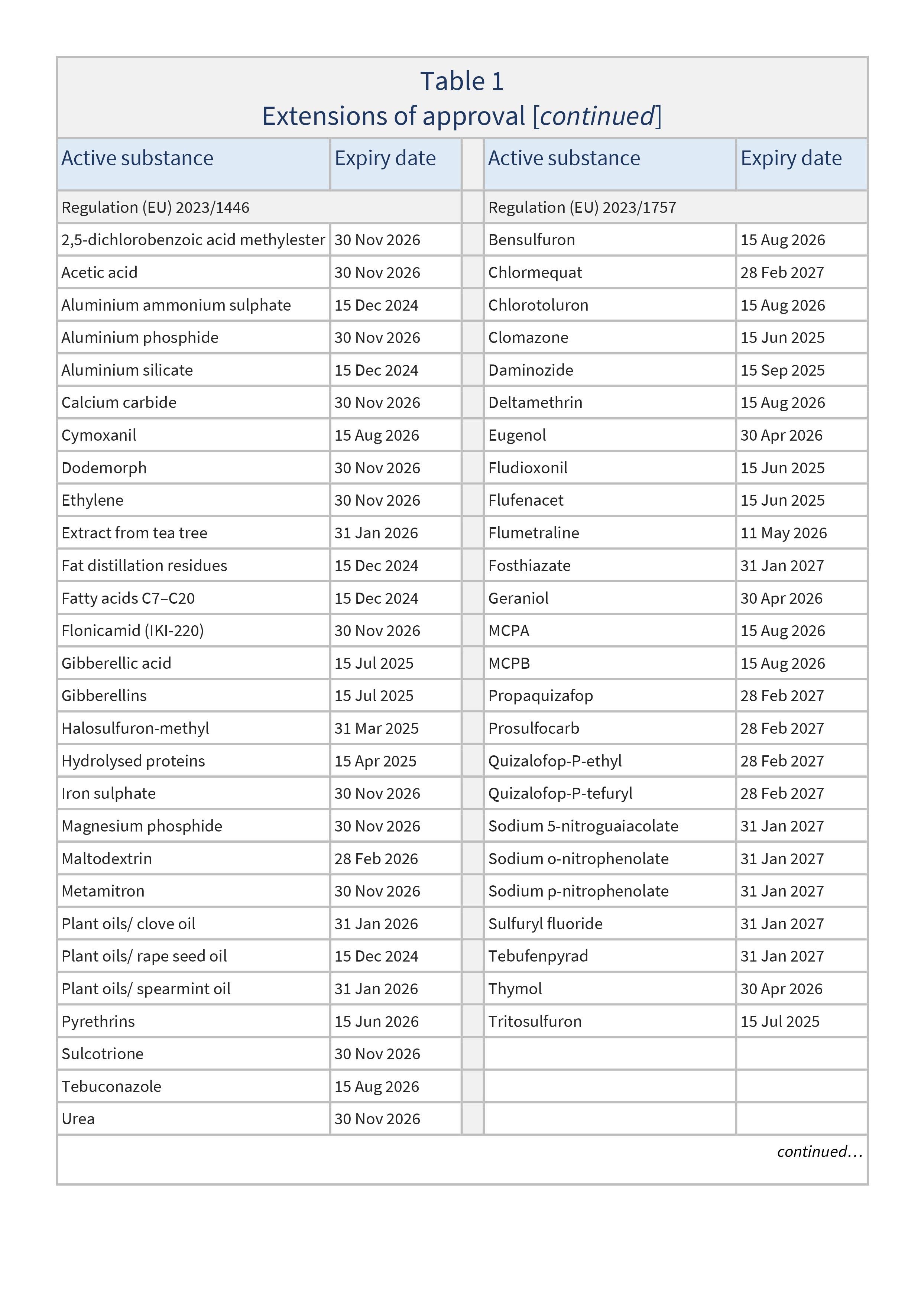
Regulation (EU) 2023/1446 as regards the extension of the approval periods of the active substances 2,5-dichlorobenzoic acid methylester, acetic acid, aluminium ammonium sulphate, aluminium phosphide, aluminium silicate, calcium carbide, cymoxanil, dodemorph, ethylene, extract from tea tree, fat distillation residues, fatty acids C7-C20, flonicamid (IKI-220), gibberellic acid, gibberellins, halosulfuron-methyl, hydrolysed proteins, iron sulphate, magnesium phosphide, maltodextrin, metamitron, plant oils/clove oil, plant oils/rape seed oil, plant oils/spear mint oil, pyrethrins, sulcotrione, tebuconazole and urea
Regulation (EU) 2023/1488 renewing the approval of the low-risk active substance quartz sand
Regulation (EU) 2023/1755 renewing the approval of the low-risk active substance fat distillation residues
Regulation (EU) 2023/1756 renewing the approval of the low-risk active substance Cydia pomonella granulovirus (CpGV)
Regulation (EU) 2023/1757 as regards the extension of the approval periods of the active substances bensulfuron, chlormequat, chlorotoluron, clomazone, daminozide, deltamethrin, eugenol, fludioxonil, flufenacet, flumetralin, fosthiazate, geraniol, MCPA, MCPB, propaquizafop, prosulfocarb, quizalofop-P-ethyl, quizalofop-P-tefuryl, sodium 5-nitroguaiacolate, sodium o-nitrophenolate, sodium p-nitrophenolate, sulfuryl fluoride, tebufenpyrad, thymol, and tritosulfuron
Regulation (EU) 2023/2589 renewing the approval of the active substance aluminium ammonium sulfate
Regulation (EU) 2023/2591 renewing the approval of the active substance ethephon
Regulation (EU) 2023/2592 as regards the extension of the approval periods of the active substances 1-naphthylacetamide, 1-naphthylacetic acid, 2-phenylphenol (incl. its salts such as sodium salt), 8-hydroxyquinoline, amidosulfuron, bifenox, dicamba, difenoconazole, diflufenican, dimethachlor, esfenvalerate, etofenprox, fenoxaprop-P, fenpropidin, fenpyrazamine, fluazifop P, lenacil, napropamide, nicosulfuron, paraffin oils, paraffin oil, penconazole, picloram, prohexadione, spiroxamine, sulphur, tetraconazole and tri-allate
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.