Ban on bisphenol A (BPA) in food packaging
- Food contact materials
- Packaging
Summary
The European Union (EU) has adopted stricter rules on the use of bisphenol A (BPA) and related chemicals in food contact materials. This is due to health concerns about the presence in food of BPA that can migrate from food packaging. This new Regulation bans the use of BPA in the manufacture of plastic food contact materials and other materials, including varnishes and coatings, printing inks, and adhesives. There are limited exceptions for the use of BPA in certain plastic film membranes and varnishes on large tanks and vessels used in food production. Where other uses are critical to the manufacture of food contact materials, authorisation may be requested.
The new rules apply from July 2026; or, in the case of single-use food contact articles intended to preserve fruit and vegetables and fishery products, from January 2028.
EU prohibits use of bisphenol A (BPA) in packaging
Commission Regulation (EU) 2024/3190 of 19 December 2024 on the use of bisphenol A (BPA) and other bisphenols and bisphenol derivatives with harmonised classification for specific hazardous properties in certain materials and articles intended to come into contact with food, amending Regulation (EU) No 10/2011 and repealing Regulation (EU) 2018/213
Update
The European Union (EU) has adopted stricter rules on the use of bisphenol A (BPA) and related chemicals in food contact materials. This is due to health concerns about the presence in food of BPA that can migrate from food packaging. This new Regulation bans the use of BPA in the manufacture of plastic food contact materials and other materials, including varnishes and coatings, printing inks, and adhesives. There are limited exceptions for the use of BPA in certain plastic film membranes and varnishes on large tanks and vessels used in food production. Where other uses are critical to the manufacture of food contact materials, authorisation may be requested.
The new rules apply from July 2026; or, in the case of single-use food contact articles intended to preserve fruit and vegetables and fishery products, from January 2028.
Impacted Products
All food produced, packaged, or stored in materials that may contain BPA or other hazardous bisphenols or their derivatives (e.g. metal food packaging such as cans, tins, and jar lids; plastic packaging including polycarbonate and polysulfone)
What is changing?
Key points
Scope: Regulation 2024/3190 (Art. 1) sets rules for the use in certain food packaging of BPA and other hazardous bisphenol derivatives that are listed in Regulation 1272/2008 (Annex VI, Part 3) as mutagenic, carcinogenic, toxic to reproduction, or endocrine disrupting for human health.
The new Regulation applies to the following categories of food contact materials:
- adhesives
- rubbers
- ion-exchange resins
- plastics
- printing inks
- silicones
- varnishes and coatings.
There are also rules on the content of BPA in food contact materials that have been manufactured using other bisphenols or their derivatives.
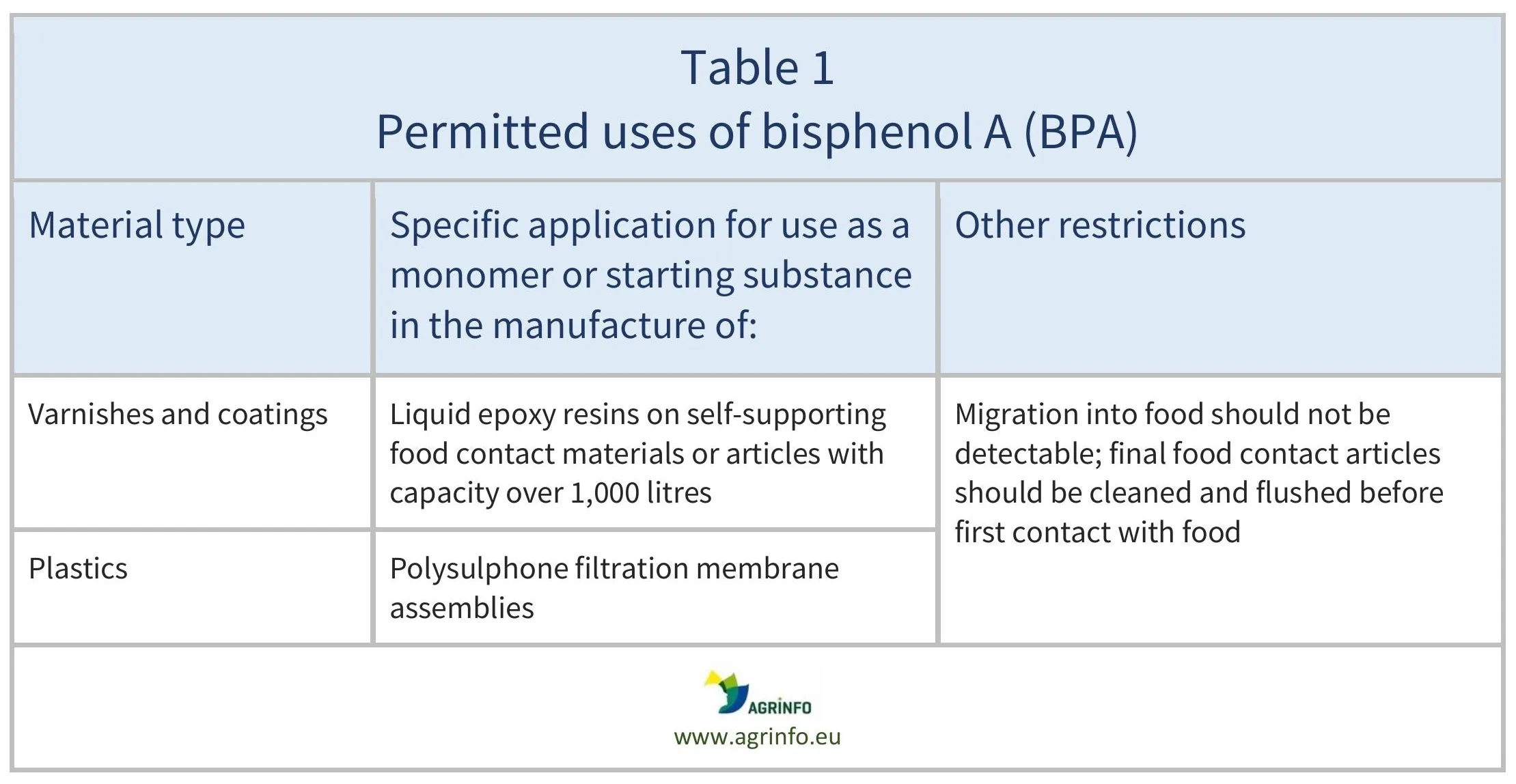
Prohibition of BPA: The use of BPA in the listed food contact material categories is prohibited (Regulation 2024/3190, Art. 3), except for the specific applications listed in Table 1 below.
No BPA residues permitted: Food contact materials manufactured using other bisphenols or bisphenol derivatives must not contain any BPA residue (Art. 4).
Other hazardous bisphenols and derivatives: These must not be used in the manufacture of food contact materials (Art. 5) unless they have been authorised, or an application for authorisation of an existing use has been submitted and certain conditions are fulfilled (see below).
Authorisation for use of hazardous bisphenols other than BPA: An application for specific use of a hazardous bisphenol other than BPA can be submitted to the European Food Safety Authority (EFSA) (Arts. 6 and 7), in accordance with Regulation 1935/2004 (Art. 9). On the basis of EFSA’s opinion, the European Commission can decide whether or not to authorise use of the hazardous bisphenol. EFSA will publish details on the information needed to support an application by early 2027.
Operators using BPA as in Table 1 below (and for subsequently authorised uses) must provide the Commission with information on the status of alternatives to the hazardous bisphenol 4 years after authorisation. Micro, small and medium-sized enterprises (see definition in European Commission 2003) do not have to report this information, but can do so on a voluntary basis.
Declaration of compliance: For foods in contact with relevant materials (plastics, varnishes/coatings, printing inks, adhesives, ion-exchange resins, rubbers), businesses at all stages in the supply chain must provide a written declaration that the materials they use are compliant (Art. 8, Annex III). This declaration of compliance should contain the:
- identity/address of the business operator issuing the declaration
- identity/address of the business operator manufacturing or importing the food contact material or article
- identity of the food contact material (including intermediate materials) and final food contact article (including packaged food)
- date of the declaration
- list of any BPA or bisphenol derivatives used in manufacturing the food contact material
- confirmation that the intermediate or final food contact material or article complies with Regulations 2024/3190 and 1935/2004 (Arts. 3, 15, 17).
Why?
Following a review, EFSA (2023) found that even minimal BPA migration into foodstuffs could exceed established tolerable daily intake (TDI) levels, with adverse effects on health, especially on the immune system.
The use of BPA in food contact materials must be minimised to protect consumers’ health. Good manufacturing practices can reduce residues of BPA to negligible amounts. However, use of BPA is permitted in some cases due to the limited availability of alternatives that can provide the same strength and chemical stability. For example, BPA can still be used in plastic separation membranes (for example those used to produce dairy-based foods), and in large containers.
Timeline
Declaration of compliance:
Since 20 January 2025, operators must ensure that the relevant food contact materials they use (see “Scope” above) are accompanied by a written declaration of compliance. If not, they should request one from their packaging suppliers.
Ban on BPA and requirements for hazardous bisphenols:
From 20 July 2026, food packaged in the relevant food contact materials that is placed on the EU market will have to comply with the ban on BPA and the new requirements for other hazardous bisphenols and derivatives.
For two exceptions (single-use food contact articles), there is a longer transition time to allow for the development of alternatives:
- packaging for fruit and vegetables, and fishery products: because the acidity of some preserved products makes it difficult to develop BPA-free alternatives
- packaging where a varnish or coating manufactured using BPA has been applied only to the outside metal surface: because the manufacture of alternatives is less advanced than for internal surfaces.
These two exceptions must comply with the new rules from 20 January 2028.
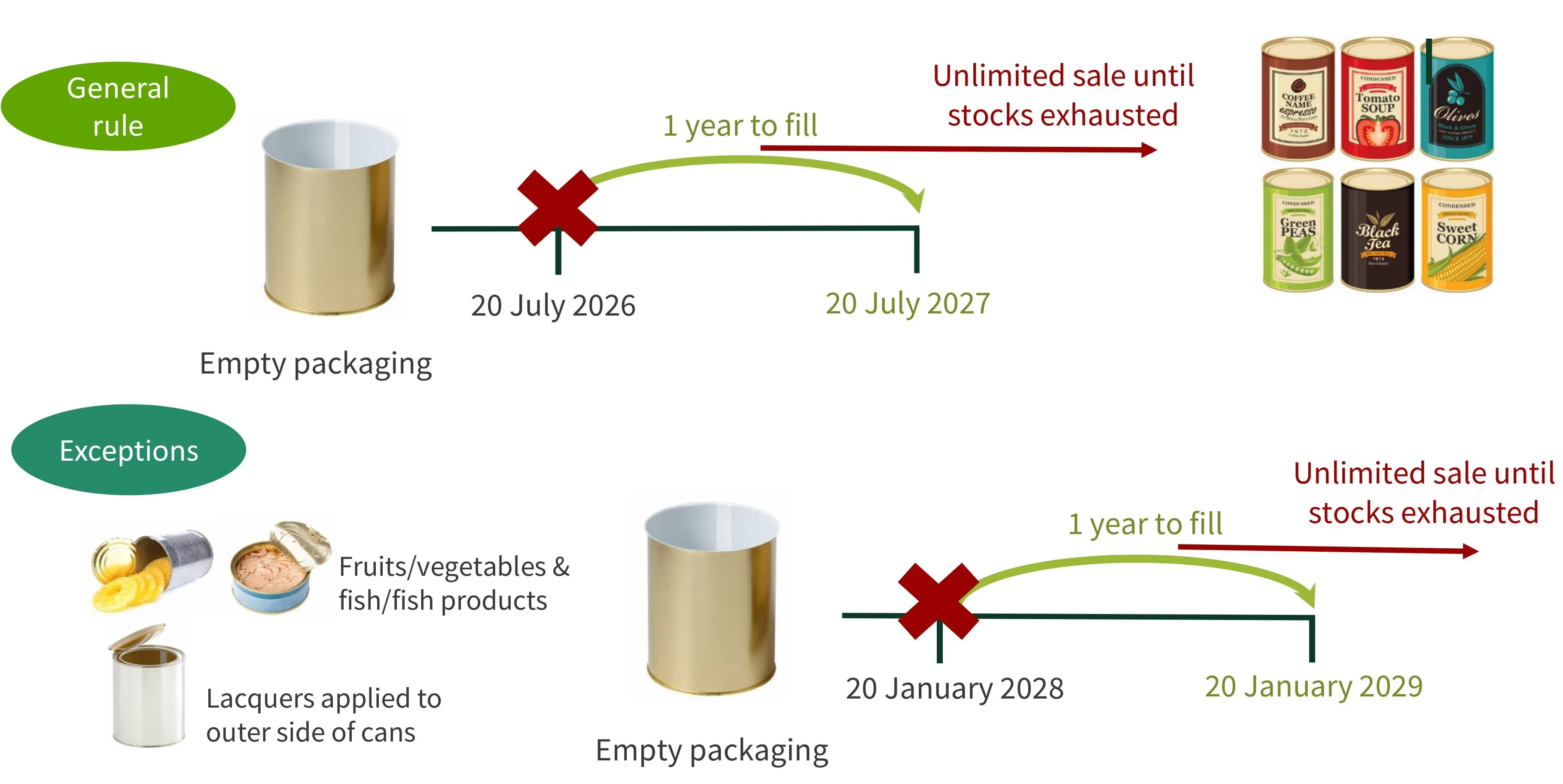
A transitional period applies to all empty packaging placed on the EU market before the application dates. Packaging can be filled with food and sealed during the 12 months after 20 July 2026 or 20 January 2028 (depending on the type of packaging). The resulting packaged food can be sold in the EU with no time limitation until stocks are exhausted.
See Figure 1 for more information.
What are the major implications for countries exporting to the EU?
Because BPA-based products are widely used, transitioning away from BPA will require careful planning to prevent supply chain disruptions. Many businesses have already started adapting to BPA-free manufacturing processes in response to demand. The transition periods included in this Regulation are intended to provide operators the time to develop alternative solutions.
The new rules will be a particular challenge for high-acidity foods such as tomatoes. Also, sourcing sufficient quantities of compliant materials will be potentially difficult in the case of seasonal foods, particularly fish products, where there is high demand for packaging in peak periods. Alternatives to BPA for varnishes and coatings used on external surfaces of metal packaging are under development.
Recommended Actions
A declaration of compliance must accompany food contact materials and articles at all stages of the supply chain.
All suppliers of packaged foods to the EU market (particularly of fruit, vegetables, and fish products) should alert their packaging suppliers to the new rules, and evaluate strategies to transition away from the use of BPA.
Background
BPA is commonly used in varnishes/coatings applied to surfaces of food packaging, such as cans, tins, or jar lids. It can be also used in materials such as printing inks and adhesives. BPA can migrate from food packaging into food.
BPA can currently be used in the manufacture of plastic food contact materials, provided a specific migration limit of 0.05 mg/kg of food is respected. The use of BPA in drinking bottles for children was already prohibited by Regulation 2018/213, which this new Regulation replaces.
Regulation 10/2011 (which is amended by this new Regulation) is one of a series of Regulations relating to specific food contact materials. It reinforces Regulation 1935/2004, which sets out the EU’s overall approach to food contact materials. For further information see Food contact materials explained.
Regulation 2023/2006 sets out general rules on good manufacturing practice related to quality assurance systems, quality control systems, and documentation. It also sets out specific rules on printing inks and quality assurance systems for plastic recycling processes.
Resources
EFSA (2023) Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Journal, 21(4): e6857.
European Commission (2003) Commission Recommendation of 6 May 2003 concerning the definition of micro, small and medium-sized enterprises.
European Commission (2013) Union Guidance on Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food as regards information in the supply chain. Updated 2016.
European Commission (2014) Union Guidelines on Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. Updated 2016.
European Commission (2015) Food contact materials.
Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food
Regulation (EU) 2018/213 on the use of bisphenol A in varnishes and coatings intended to come into contact with food
Regulation EU No 321/2011 as regards the restriction of use of Bisphenol A in plastic infant feeding bottles
Sources
Commission Regulation (EU) 2024/3190 on the use of bisphenol A (BPA) and other bisphenols and bisphenol derivatives with harmonised classification for specific hazardous properties in certain materials and articles intended to come into contact with food
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU prohibits use of bisphenol A (BPA) in packaging
Commission Regulation (EU) 2024/3190 on the use of bisphenol A (BPA) and other bisphenols and bisphenol derivatives with harmonised classification for specific hazardous properties in certain materials and articles intended to come into contact with food
What is changing and why?
The European Food Safety Authority (EFSA) has identified health risks from levels of bisphenol A (BPA) and related substances in food. The EU has therefore adopted a new Regulation that:
- bans use of BPA in the manufacture of food contact materials including plastics, varnishes/coatings, printing inks, adhesives, ion-exchange resins, and rubbers
- allows exceptional use of BPA in certain plastic film membranes and varnishes used on large tanks
- allows operators to request authorisation of other use of BPA
- requires food businesses to prove their compliance with the BPA Regulation for all relevant packaged food placed on the EU market.
The European Commission aims to safeguard consumer health by minimising exposure to BPA and raising safety standards in the production of food contact materials.
Actions
A declaration of compliance must accompany food contact materials and articles at all stages of the supply chain.
All suppliers of packaged foods to the EU market (particularly of fruit, vegetables, and fish products) should alert their packaging suppliers to the new rules, and evaluate strategies to transition away from the use of BPA.
Timeline
Declaration of compliance:
Since 20 January 2025, operators must ensure that the relevant food contact materials they use are accompanied by a written declaration of compliance. If not, they should request one from their packaging suppliers.
Ban on BPA and requirements for hazardous bisphenols:
From 20 July 2026, food packaged in the relevant food contact materials that is placed on the EU market will have to comply with the ban on BPA and the new requirements for other hazardous bisphenols and derivatives.
For two exceptions (single-use “food contact articles”), there is a longer transition time to allow for the development of alternatives:
- packaging for fruit and vegetables, and fishery products: because the acidity of some preserved products makes it difficult to develop BPA-free alternatives
- packaging where a varnish or coating manufactured using BPA has been applied only to the outside metal surface: because the manufacture of alternatives is less advanced than for internal surfaces.
These two exceptions must comply with the new rules from 20 January 2028.
A transitional period applies to all empty packaging placed on the EU market before the application dates. Packaging can be filled with food and sealed during the 12 months after 20 July 2026 or 20 January 2028 (depending on the type of packaging). The resulting packaged food can be sold in the EU with no time limitation until stocks are exhausted.
See Figure 1 for more information.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.