Biocides explained
- Biocides
- Food safety
Summary
The Biocidal Products Regulation is the legal framework for the authorisation and use of biocidal products, which include household disinfectants, insecticides, and other chemicals used to control pests (parasites, fungi, bacteria, etc.) or to protect materials. These products, commonly used in the food chain, can pose risks to humans, animals and the environment.
Background information on the Biocidal Products Regulation
Regulation (EU) 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products
Update
The Biocidal Products Regulation is the legal framework for the authorisation and use of biocidal products, which include household disinfectants, insecticides, and other chemicals used to control pests (parasites, fungi, bacteria, etc.) or to protect materials. These products, commonly used in the food chain, can pose risks to humans, animals and the environment.
Background
Substances used to suppress, eradicate and prevent organisms that are considered harmful are grouped under the term “pesticide”. The term includes both plant protection products (used on plants in agriculture, horticulture, parks and gardens) and biocidal products (used in other applications, for example, as a disinfectant or to protect materials) (European Parliament 2017).
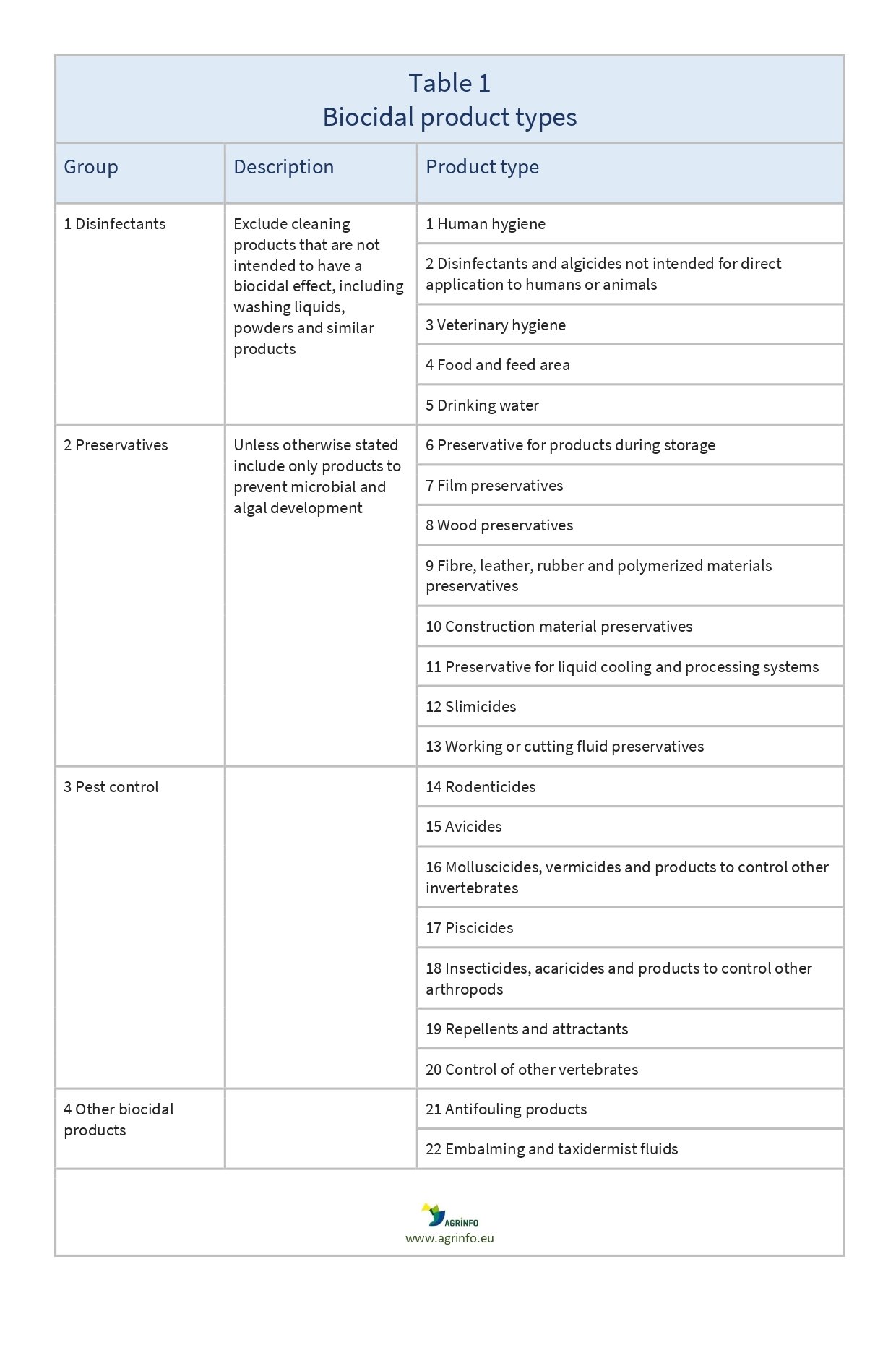
Biocides are typically classified into four groups based on their intended use (see Table 1).
- Disinfectants: includes biocides used to kill or inactivate microorganisms on inanimate objects, e.g. chlorine, hydrogen peroxide, quaternary ammonium compounds.
- Protective products: includes biocides used to protect materials from the harmful effects of microorganisms, e.g. wood preservatives, paint additives, fabric protectants.
- Products for control of so-called "harmful" species: includes biocides used to control pests such as insects, rodents and plant diseases, e.g. molluscicides (used to control snails and slugs), algicides, bactericides.
- Other biocidal products: includes biocides that do not fit into the other three categories.
Like plant protection products, biocidal products are approved in two stages. First, the active substance is evaluated by an EU Member State "rapporteur", who produces an assessment report that is reviewed by other Member States in the Biocidal Products Committee of the European Chemicals Agency (ECHA). The Commission’s decision to approve is based on the ECHA’s opinion. The approval of an active substance is granted for a defined number of years, not exceeding 10 years, and is renewable. Second, companies may apply for permission to place their products containing the active substance on the market, by an authorisation granted either by the European Commission, or by a Member State.
The EU sets MRLs for biocides that are used in or on food and animal feed, as well as for biocides that are used in other non-food applications. The MRLs are set at levels considered to be safe for consumers, taking into account potential exposure to biocide residues through dietary and other sources.
What are the major implications for exporting countries?
Agri-food suppliers in non-EU countries may face problems exporting to the EU if biocidal products used when supplying food (e.g. during storage, transport or processing) are not authorised in the EU for a specific use, or are not used correctly. Agri-food processors may also face challenges in ensuring that biocidal products used in packaging material do not pose a risk to food safety.
Any residues from biocidal products must conform with EU pesticide MRLs. Where a food is found to contain a residue that exceeds an EU MRL, the food will be non-compliant with EU law, even if no pesticide was used and the residue comes from a biocidal product.
For example, recently there have been problems with residues of chlorates in food. Residues are frequently found that are the result of by-products of chlorine solutions used as sanitising and disinfection agents in the food industry. MRLs for chlorate apply even where residues are not linked to their direct use on the food product, and can lead to food safety recalls or other regulatory action (EFSA 2020).
Contamination can result from poor rinsing of materials or packaging after the use of biocides. For example, disinfected milk tanks, fixed sprayers for vegetables in greenhouses, and small pots used for baby foods can all pose a risk to human health.
The use of biocidal products before processing, such as blanching of vegetables, can also result in the contamination of food products and pose potential health risks to consumers.
Recommended Actions
Agri-food processors must ensure that they use any biocidal products safely and appropriately, and that they keep any residues to a minimum. One strategy to minimise the risk of illegal contamination of food is to use biocides containing only active substances that are authorised in the EU.
The approval status of substances used in biocidal products can be checked on the European Chemicals Agency (ECHA) Biocides database.
Suppliers must ensure that any residues in food comply with the maximum residue levels (MRLs) established for pesticides. that can be found in the EU Pesticide Residues database.
Resources
EFSA (2020) The 2018 European Union report on pesticide residues in food.
European Parliament (2017) EU policy and legislation on pesticides: Plant protection products and biocides.
Sources
Regulation (EU) 528/2012 concerning biocidal products
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
