Consultation on maximum levels for some contaminants in feed
- Feed safety
Summary
The EU has informed the World Trade Organization Sanitary and Phytosanitary Measures (WTO SPS) Committee that it proposes to establish maximum levels for arsenic, cadmium, lead, nickel, rye ergot, delta-9-tetrahydrocannabinol, endosulfan, heptachlor, hexachlorbenzene, hexachlorohexane, dioxins and PCBs, Datura sp., certain coccidiostats and histomonostats, and p-phenetidine in animal feed (G/SPS/N/EU/703).
EU proposes to set and amend maximum levels for some contaminants in feed
Draft Commission delegated Regulation amending Annexes I and II to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels and action thresholds for arsenic, cadmium, lead, nickel, rye ergot, delta-9-tetrahydrocannabinol, endosulfan, heptachlor, hexachlorbenzene, hexachlorohexane, dioxins and PCBs, Datura sp., certain coccidiostats and histomonostats and p-phenetidine in animal feed
Update
The EU has informed the World Trade Organization Sanitary and Phytosanitary Measures (WTO SPS) Committee that it proposes to establish maximum levels for arsenic, cadmium, lead, nickel, rye ergot, delta-9-tetrahydrocannabinol, endosulfan, heptachlor, hexachlorbenzene, hexachlorohexane, dioxins and PCBs, Datura sp., certain coccidiostats and histomonostats, and p-phenetidine in animal feed (G/SPS/N/EU/703).
Impacted Products
Feed
What is changing?
The main proposed changes to permitted levels of these contaminants in animal feed are:
- establishment of maximum levels for nickel, Δ9-tetrahydrocannabinol (Δ9-THC), and p-phenetidine
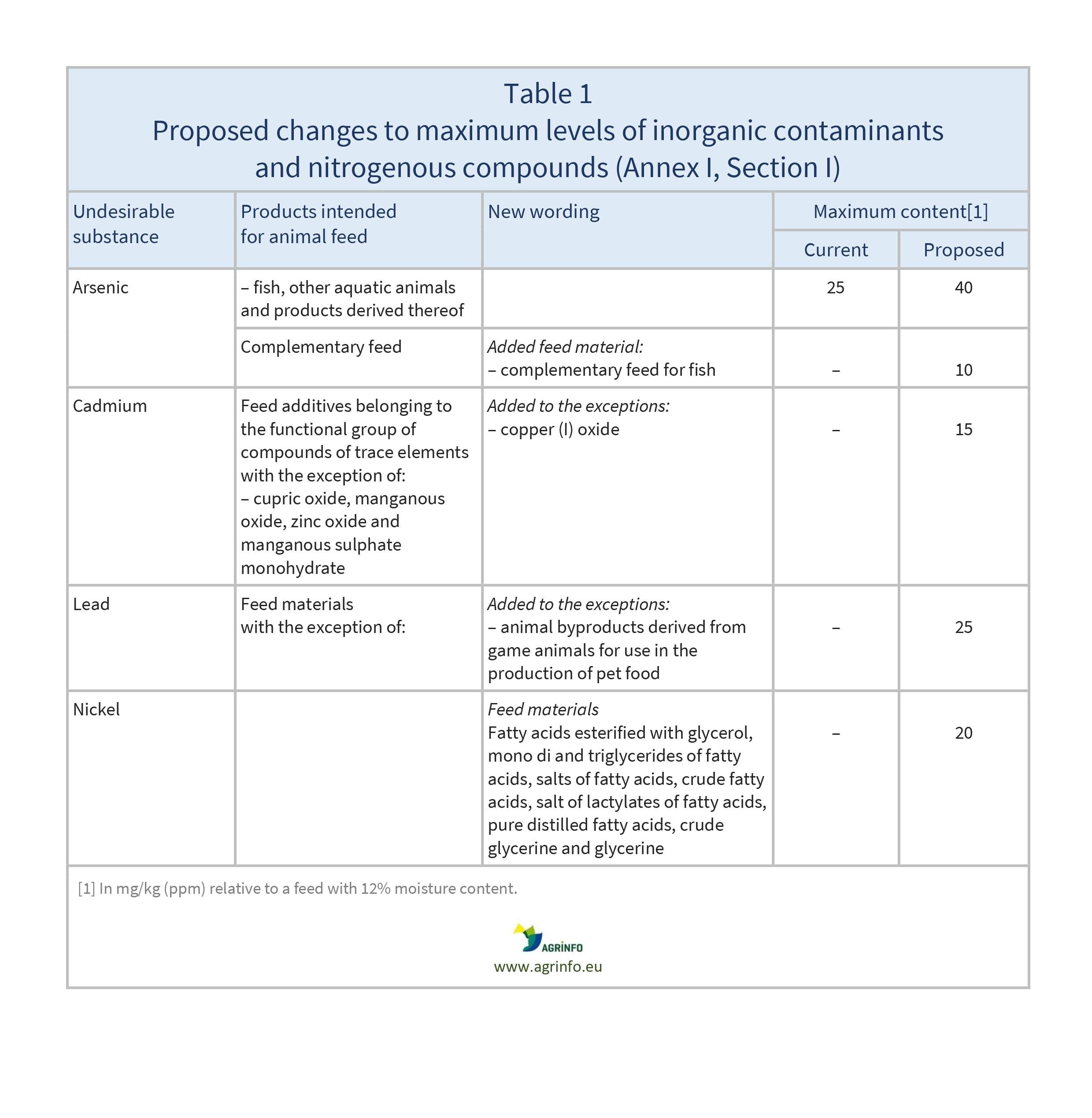
- increase of maximum levels for arsenic in fish feed, cadmium in copper (I) oxide, and lead in game meat for use in pet food
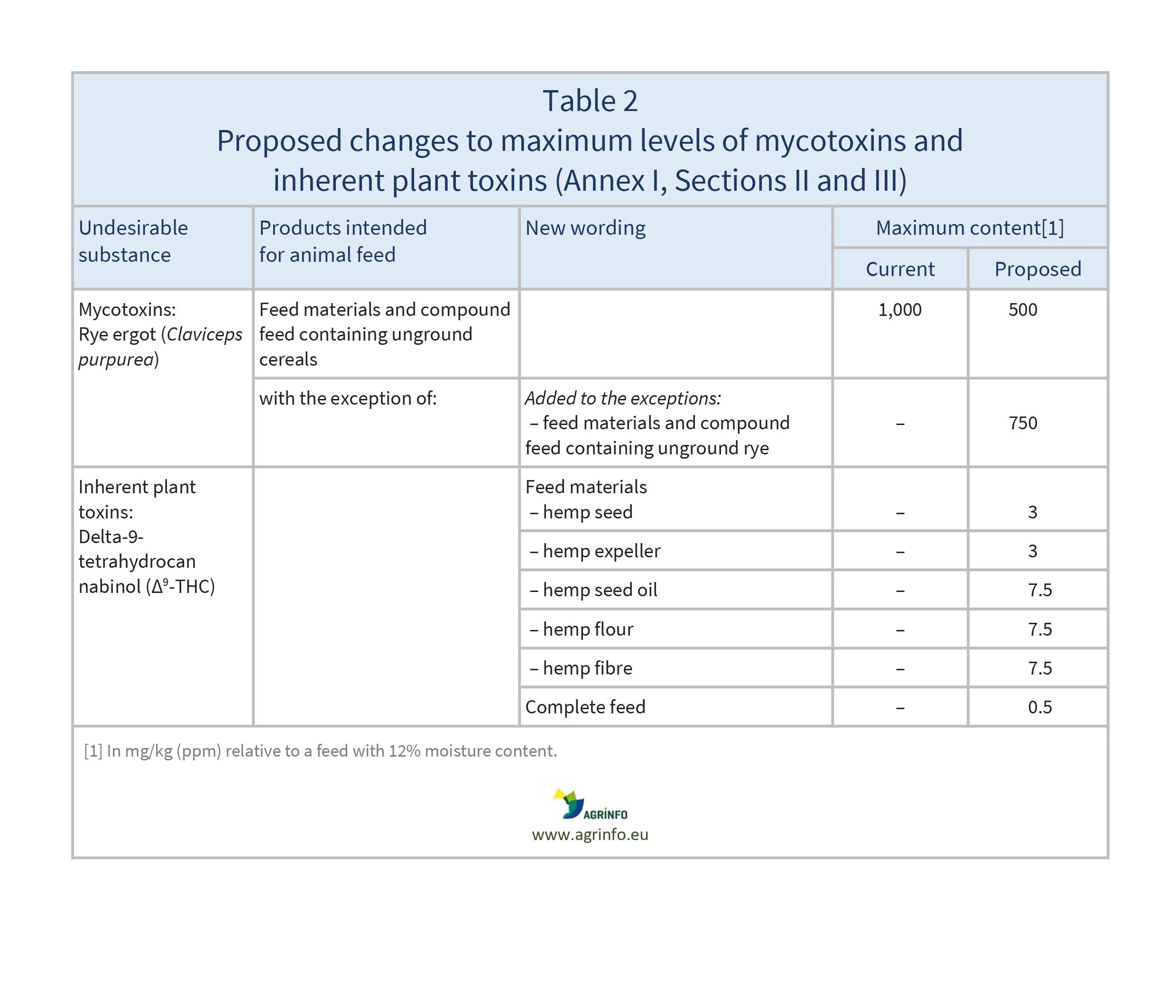
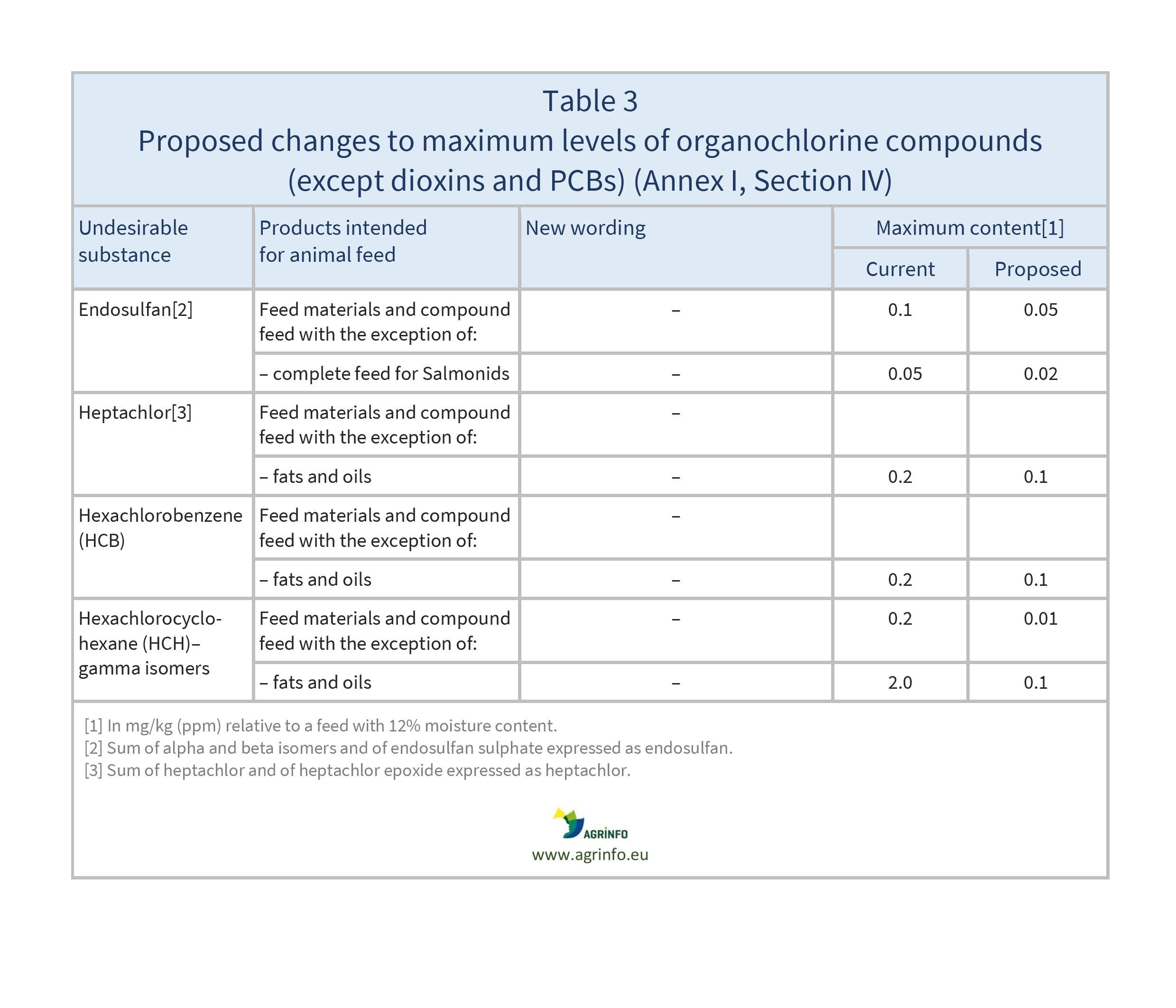
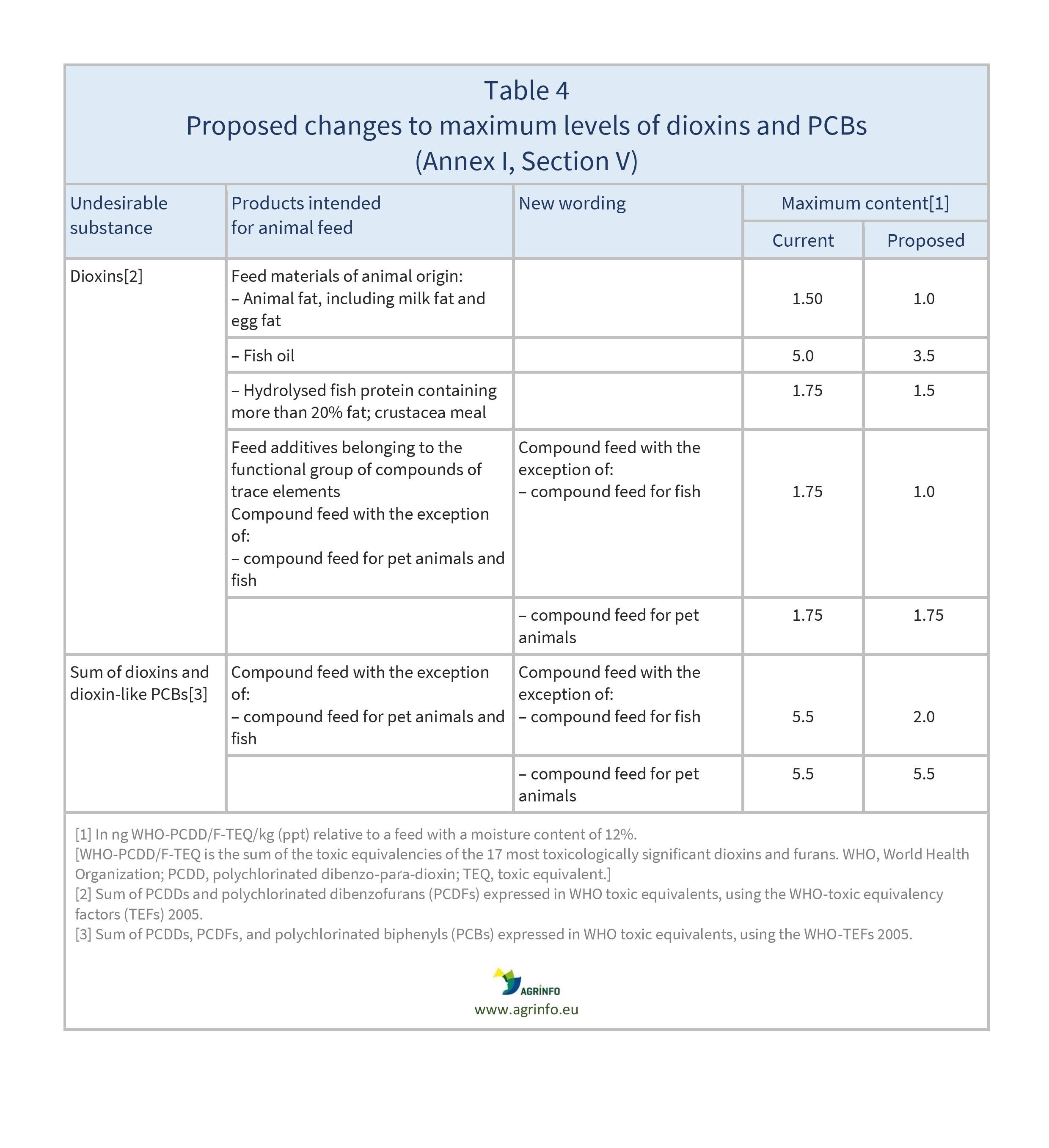
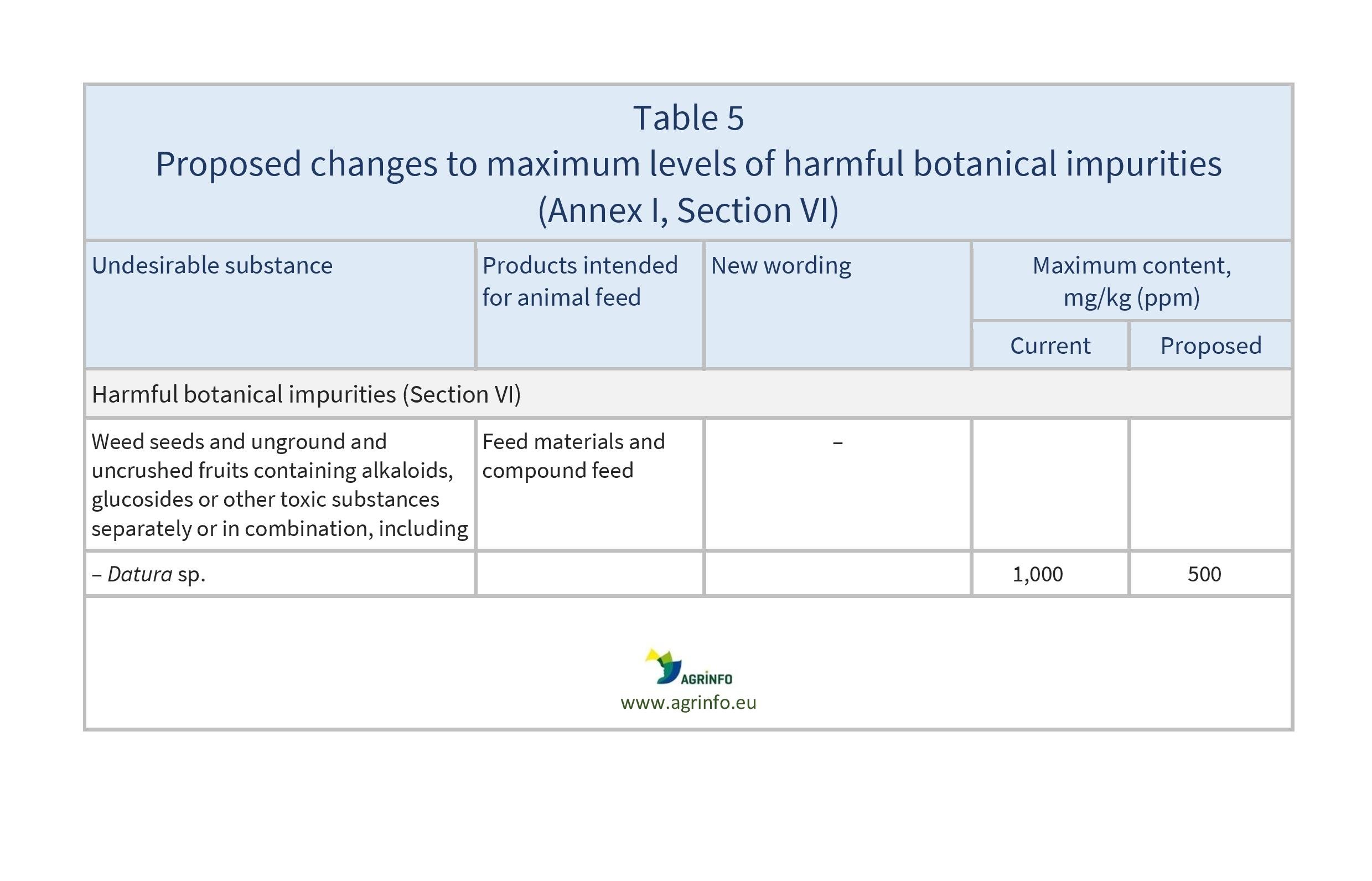
- lowering of maximum levels for rye ergot, endosulfan, heptachlor, hexachlorobenzene, gamma-hexachlorocyclohexane, dioxins and dioxin-like PCBs, and Datura seeds
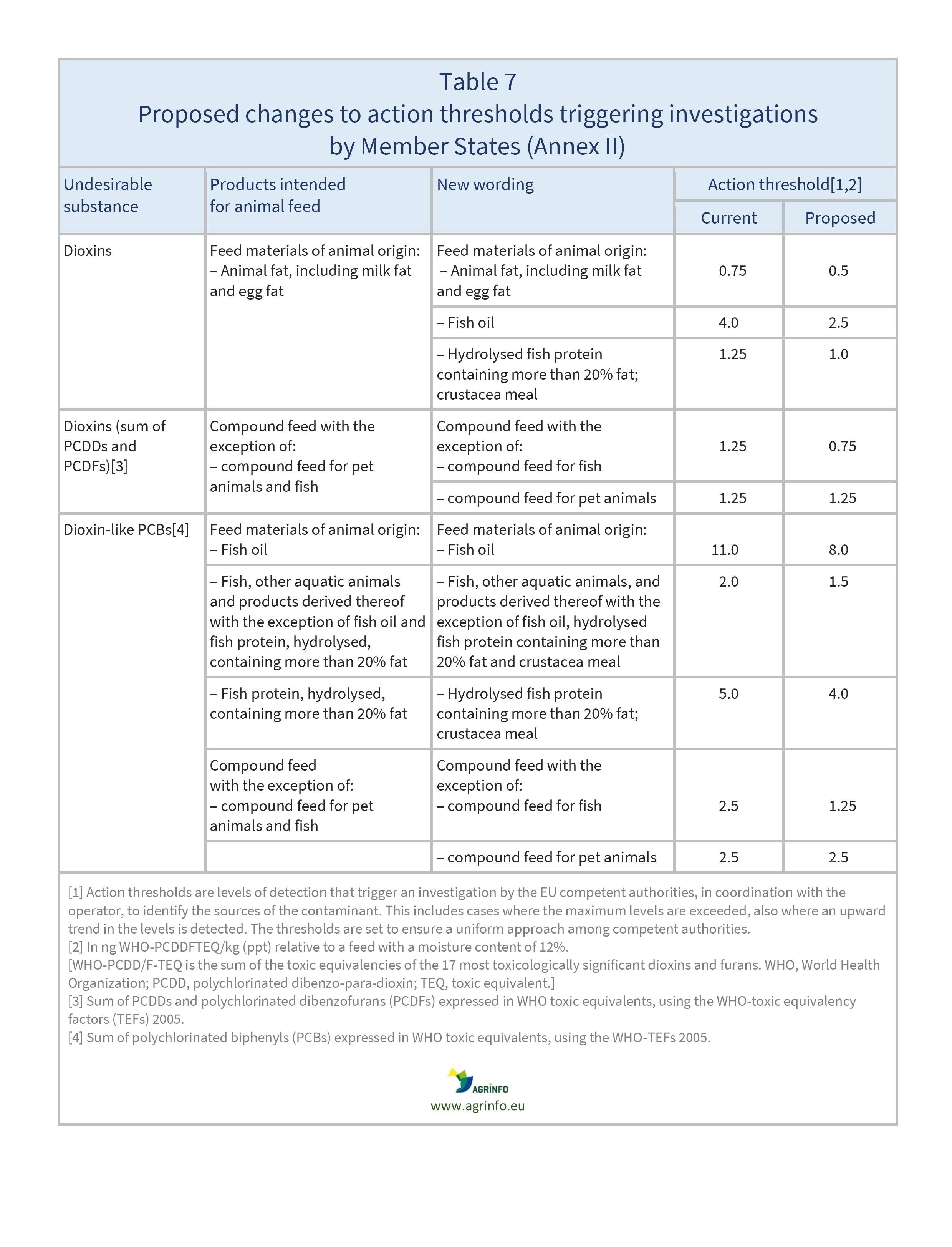
- changes to certain action levels for dioxins and PCBs.
In addition, there are amendments to some wording of the feed materials, e.g. “cupric acid” is replaced by “copper (II) oxide”. See the proposed Annexes to the Regulation for details.
Maximum levels
“Maximum levels” here refer to the maximum levels of contaminants that can be accepted in a given product or products. The changes to maximum levels proposed in Annex I are highlighted in the Tables below.
- Table 1: inorganic contaminants and nitrogenous compounds (Section I)
- Table 2: mycotoxins and inherent plant toxins (Sections II and III)
- Table 3: organochlorine compounds (except dioxins and PCBs) (Section IV)
- Table 4: dioxins and PCBs (Section V)
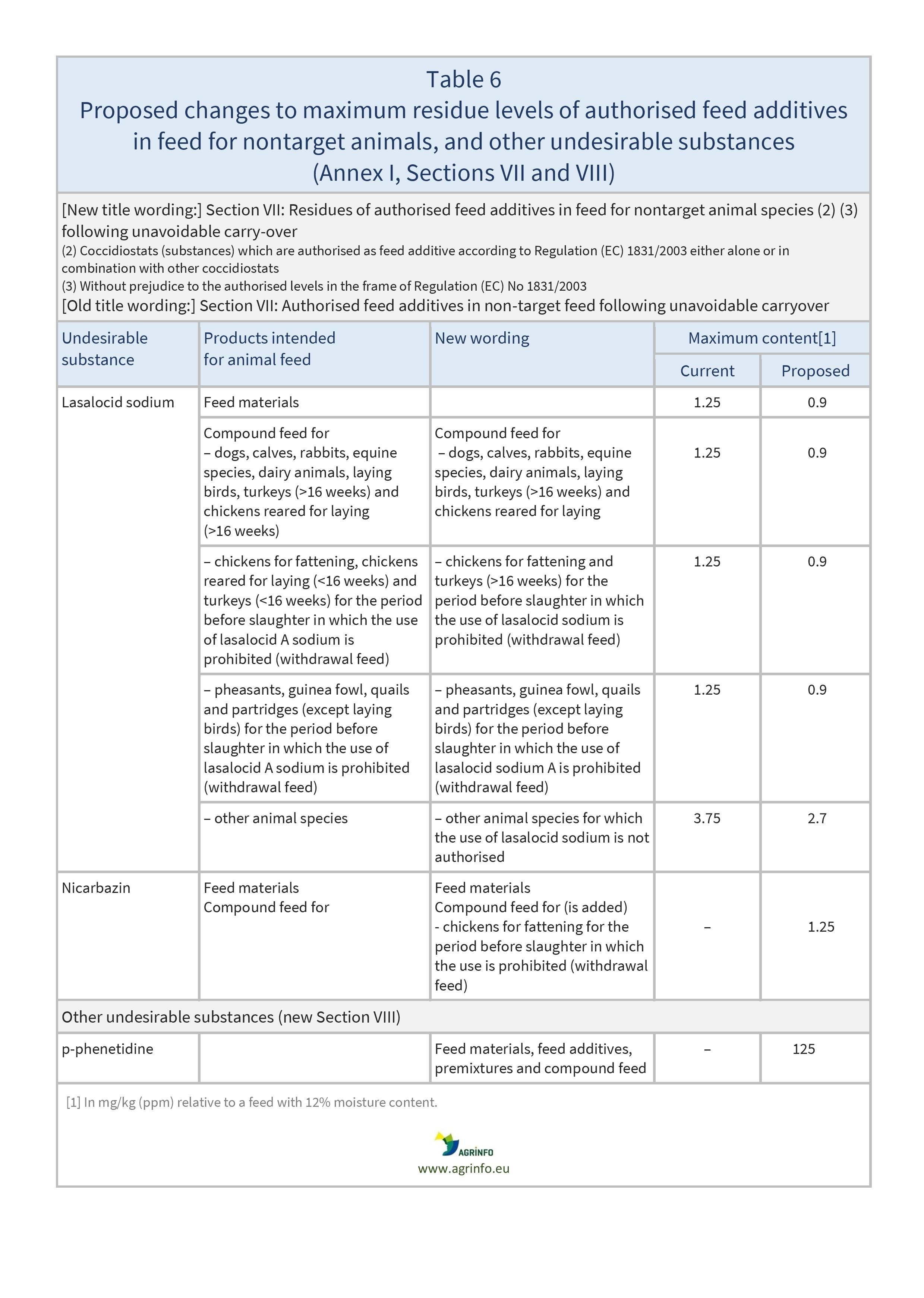
- Table 5: residues of authorised feed additives in feed for nontarget animals (Section VII)
- Table 6: harmful botanical impurities and other undesirable substances (Sections VI and VIII).
In addition, Table 7 shows proposed changes to action thresholds triggering investigations by Member States (Annex II).
Why?
The proposed changes to maximum levels respond to a series of evaluations undertaken by the European Food Safety Authority aimed at ensuring the levels are achievable, while preserving animal and human health (EFSA 2005a, 2005b, 2006, 2007, 2008, 2011, 2018, 2022).
Timeline
The Regulation is expected to apply 20 days following that of its publication in the Official Journal of the European Union.
Recommended Actions
Suppliers of feed materials to the EU market should check their compliance with the new proposed maximum levels of contaminants, and where necessary take steps to ensure compliance by July 2024.
Background
Maximum levels and action thresholds for undesirable substances in feed were established by Directive 2002/32/EC.
Resources
Directive 2002/32 on undesirable substances in animal feed
EFSA (2005a) Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to endosulfan as undesirable substance in animal feed. EFSA Journal, 3(7): 234.
EFSA (2005b) Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to gamma-hexachlorocyclohexane (𝛾-HCH) and other hexachlorocyclohexanes as undesirable substance in animal feed. EFSA Journal, 3(7): 250.
EFSA (2006) Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to hexachlorobenzene as undesirable substance in animal feed. EFSA Journal, 4(10): 402.
EFSA (2007) Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to heptachlor as undesirable substance in animal feed. EFSA Journal, 5(6): 478.
EFSA (2008) Tropane alkaloids (from Datura sp.) as undesirable substances in animal feed[1] – Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA Journal, 6(8): 691.
EFSA (2011) Scientific Opinion on the safety of hemp (Cannabis genus) for use as animal feed. EFSA Journal, 9(3): 2011.
EFSA (2018) Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA Journal, 16(11): 5333.
EFSA (2022) Safety and efficacy of a feed additive consisting of ethoxyquin (6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline) for all animal species (FEFANA asbl). EFSA Journal, 20(3): 7166.
Sources
Draft Regulation as regards maximum levels and action thresholds for arsenic, cadmium, lead, nickel, rye ergot, delta-9-tetrahydrocannabinol, endosulfan, heptachlor, hexachlorbenzene, hexachlorohexane, dioxins and PCBs, Datura sp., certain coccidiostats and histomonostats and p-phenetidine in animal feed
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU proposes to set and amend maximum levels for some contaminants in feed
Draft Regulation as regards maximum levels and action thresholds for arsenic, cadmium, lead, nickel, rye ergot, delta-9-tetrahydrocannabinol, endosulfan, heptachlor, hexachlorbenzene, hexachlorohexane, dioxins and PCBs, Datura sp., certain coccidiostats and histomonostats and p-phenetidine in animal feed
What is changing and why?
The EU is proposing to change the maximum permitted levels of some contaminants in animal feed, and certain action thresholds triggering investigations by Member States. The details of these changes are shown in the Tables below. This proposal is based on experience of contaminant levels under the current Regulations, and the results of evaluations conducted by the European Food Safety Authority (EFSA). It aims to ensure that the new levels are achievable, while preserving animal and human health.
Actions
Suppliers of feed materials to the EU market should check their compliance with the new proposed maximum levels of contaminants, and where necessary take steps to ensure compliance by July 2024.
Timeline
The Regulation is expected to apply 20 days following that of its publication in the Official Journal of the European Union.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.