Feed additives: January–February 2024 authorisations
- Feed additives
- Feed safety
Summary
An overview of the latest authorisations, reauthorisations, and denied authorisations of feed additives and their use in animal nutrition in target animals.
EU authorises or reauthorises certain feed additives, denies other authorisations
Commission Implementing Regulations 2024/220, 2024/221, 2024/228, 2024/231, 2024/239, 2024/251, 2024/252, 2024/260, 2024/261, 2024/262, 2024/265, 2024/285, 2024/749, 2024/750, 2024/752, 2024/754, 2024/763
Update
An overview of the latest authorisations, reauthorisations, and denied authorisations of feed additives and their use in animal nutrition in target animals.
Impacted Products
Feed additives, prepared fodder
What is changing?
New authorisations (January - February 2024)
In January and February 2024, the EU authorised the new feed additives listed in Table 1.
These authorisations are based on the following opinions published by the European Food Safety Authority: EFSA (2022a), (2022b), (2022c), (2022d), (2022e), (2023a), (2023b), (2023c), (2023d), (2023e), (2023f), (2023g), (2023h), (2023i).
Reauthorisations
In January and February 2024, the EU reauthorised the feed additives listed in Table 2.
These reauthorisations are based on the following opinions published by EFSA: (2023j), (2023k), (2023l), (2023m), (2023n), (2023o), (2023p), (2023q).
Denied authorisations
Regulation 2024/752 denies authorisation of a preparation of astaxanthin-rich Phaffia rhodozyma (ATCC SD-5340) as a feed additive for salmon and trout, based on EFSA’s opinion (2022f).
Amended authorisations
Regulation 2024/754 authorises the use of a preparation of Bacillus subtilis DSM 32324, DSM 32325 and Bacillus amyloliquefaciens DSM 25840 for all poultry species simultaneously with the coccidiostats diclazuril, decoquinate, halofuginone, monensin, salinomycin, narasin, a combination of nicarbazin and narasin, and lasalocid, based on EFSA’s opinion (2023r) that these uses are compatible.
The EU also corrected and amended Implementing Regulations (EU) 2022/1421, (EU) 2022/652, (EU) 2022/1490 and (EU) 2022/320 regarding the use of expressed orange essential oil, distilled orange essential oil and folded orange oils from Citrus sinensis as feed additives for dogs, cats, horses, rabbits, ornamental fish, and ornamental birds.
Why?
Applications for the above authorisations and reauthorisations were submitted and considered by the Reference Laboratory set up by the Feed Additives Regulation (1831/2003).
EFSA could not conclude on the safety of astaxanthin-rich Phaffia rhodozyma (ATCC SD-5340) as a feed additive for salmon and trout, and its safety for consumers of these products.
EFSA could not conclude on the safety of citrus by-products as feed additives for dogs, cats and ornamental fish, because they are not normally exposed to those by-products.
Timeline
Regulations 2024/220, 2024/221, 2024/228, 2024/231, and 2024/260 apply from 1 February 2024. These authorisations remain valid until 4 February 2034.
Regulations 2024/251, 2024/252, and 2024/285 apply from 6 February 2024. These authorisations remain valid until 6 February 2034.
Regulations 2024/261, 2024/262, 2024/265, and 2024/285 apply from 7 February 2024. These authorisations remain valid until 7 February 2034.
Regulations 2024/749, 2024/750, 2024/752, 2024/754, and 2024/763 will apply from 21 March 2024. These authorisations remain valid until 21 March 2034.
What are the major implications for exporting countries?
With new authorisations, more feed additives will be available on the market. Authorisations and renewals are valid for 10 years. The way that all preparations and substances specified as feed additives are used must comply with the provisions of use specified in the Annex to each Regulation.
Recommended Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of the feed additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel format (see foot of Food and Feed Information Portal).
Background
The procedure for authorising the placing on the market and use of feed additives is set out in Regulation (EC) 1831/2003. For the latest updates on feed additives see the EU Feed Additives register.
Resources
European Commission:
Regulation 1831/2003 on additives for use in animal nutrition
European Food Safety Authority:
EFSA (2023a) Efficacy of a feed additive consisting of Companilactobacillus farciminis CNCM I-3740 (Biacton®) for chickens and turkeys for fattening (ChemVet dk A/S). EFSA Journal, 21(6): 8049.
EFSA (2023b) Safety and efficacy of a feed additive consisting of halofuginone hydrobromide (STENOROL®) for chickens for fattening and turkeys for fattening/reared for breeding. EFSA Journal, 21(4): 7978.
EFSA (2023c) Safety and efficacy of a feed additive consisting of a tincture derived from the fruit of Anethum graveolens L. (dill tincture) for use in all animal species (FEFANA asbl). EFSA Journal, 21(1): 7691.
EFSA (2023d) Safety and efficacy of a feed additive consisting of a tincture derived from the fruit of Foeniculum vulgare Mill. ssp. vulgare var. dulce (sweet fennel tincture) for use in all animal species (FEFANA asbl). EFSA Journal, 21(1): 7693.
EFSA (2023e) Safety and efficacy of a feed additive consisting of a tincture derived from the fruit of Petroselinum crispum (Mill.) Fuss (parsley tincture) for use in all animal species (FEFANA asbl). EFSA Journal, 21(1): 7694.
EFSA (2023f) Safety and efficacy of feed additives prepared from Piper nigrum L.: black pepper oil and black pepper oleoresin for use in all animal species and a supercritical extract for use in dogs and cats (FEFANA asbl). EFSA Journal, 20(11): 7599.
EFSA (2023g) Safety and efficacy of a feed additive consisting of a zinc(II)–betaine complex for all animal species (Biochem Zusatzstoffe Handels- und Produktionsges. mbH). EFSA Journal, 21(2): 7819.
EFSA (2023h) Safety and efficacy of a feed additive consisting of a tincture derived from the roots of Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (taiga root tincture) for use in dogs, cats and horses (FEFANA asbl). EFSA Journal, 21(2): 7876.
EFSA (2023i) Safety of a feed additive consisting of lignosulphonate for all animal species (Borregaard AS). EFSA Journal, 21(4): 7956.
EFSA (2023j) Assessment of the feed additive consisting of Pediococcus pentosaceus NCIMB 30168 for all animal species for the renewal of its authorisation (Volac International Ltd). EFSA Journal, 21(6): 8046.
EFSA (2023k) Safety and efficacy of a feed additive consisting of endo-1,4-beta-xylanase, endo-1,3(4)-beta-glucanase and endo-1,4-beta-glucanase produced by Trichoderma reesei ATCC 74444 (Ronozyme® Multigrain) for use in poultry for fattening, poultry for laying and piglets (weaned) (DSM Nutritional Products). EFSA Journal, 21(6): 8043.
EFSA (2023m) Assessment of the feed additive consisting of Lactiplantibacillus plantarum DSM 23375 for all animal species for the renewal of its authorisation (Agri-King, Inc.). EFSA Journal, 21(6): 8054.
EFSA (2023n) Safety and efficacy of a feed additive consisting of endo-1,4-β-glucanase produced by Trichoderma citrinoviride IMI 360748 (Hostazym C) for use in all poultry species for fattening and reared for laying/breeding, ornamental birds and piglets (weaned and suckling) (Huvepharma NV). EFSA Journal, 21(4): 7954.
EFSA (2023o) Assessment of the feed additive consisting of thaumatin for all animal species for the renewal of its authorisation (ADISSEO France S.A.S.). EFSA Journal, 21(6): 8077.
EFSA (2023p) Assessment of the feed additive consisting of Lactiplantibacillus plantarum (formerly Lactobacillus plantarum) NCIMB 30083 for all animal species for the renewal of its authorisation (Chr. Hansen A/S). EFSA Journal, 21(8): 8154.
EFSA (2023q) Assessment of the feed additive consisting of Lactiplantibacillus plantarum (formerly Lactobacillus plantarum) NCIMB 30084 for all animal species for the renewal of its authorisation (Chr. Hansen A/S). EFSA Journal, 21(7): 8167.
EFSA (2023r) Assessment of the application for modification of the terms of the authorisation of the feed additive consisting of Bacillus subtilis DSM 32324, Bacillus subtilis DSM 32325 and Bacillus amyloliquefaciens DSM 25840 (GalliPro® Fit) for all poultry species for fattening and reared for laying/breeding (Chr. Hansen A/S). EFSA Journal, 21(8): 8179.
EFSA (2022a) Safety and efficacy of a feed additive consisting of an essential oil from the fruit of Cuminum cyminum L. (cumin oil) for use in all animal species (FEFANA asbl). EFSA Journal, 20(12): 7690.
EFSA (2022b) Safety and efficacy of a feed additive consisting of a tincture derived from the roots of Angelica sinensis (Oliv.) Diels (dong quai tincture) for use in poultry, horses, dogs and cats (FEFANA asbl). EFSA Journal, 20(12): 7692.
EFSA (2022c) Safety and efficacy of a feed additive consisting of a tincture derived from the fruit of Illicium verum Hook f. (star anise tincture) for use in all animal species (FEFANA asbl). EFSA Journal, 20(12): 7695.
EFSA (2022d) Safety and efficacy of a feed additive consisting of an essential oil from the aerial parts of Anethum graveolens L. (dill herb oil) for use in dogs and cats (FEFANA asbl). EFSA Journal, 20(12): 7689.
EFSA (2022e) Safety and efficacy of a feed additive consisting of an essential oil from the gum resin of Ferula assa-foetida L. (asafoetida oil) for use in dogs and cats (FEFANA asbl). EFSA Journal, 20(12): 7688.
EFSA (2022f) Safety and efficacy of a feed additive consisting of astaxanthin‐rich Phaffia rhodozyma for salmon and trout (Igene Biotechnology, Inc.). EFSA Journal, 20(2): 7161.
Sources
Commission Implementing Regulations:
2024/220 concerning the renewal of the authorisation of a preparation of Pediococcus pentosaceus NCIMB 30168 as a feed additive for all animal species
2024/221 concerning the renewal of the authorisation of a preparation of endo-1,4-beta-xylanase, endo-1,3(4)-beta-glucanase and endo-1,4-beta-glucanase, produced by Trichoderma reesei ATCC 74444, as a feed additive for all poultry species for fattening, all poultry species for laying and weaned piglets
2024/228 concerning the authorisation of a preparation of Companilactobacillus farciminis CNCM I-3740 as a feed additive for chickens for fattening and turkeys for fattening
2024/231 concerning the authorisation of a preparation of halofuginone hydrobromide (Stenorol) as a feed additive for chickens for fattening, turkeys for fattening and turkeys reared for breeding
2024/239 correcting and amending Implementing Regulations (EU) 2022/1421, (EU) 2022/652, (EU) 2022/1490 and (EU) 2022/320
2024/251 concerning the renewal of the authorisation of the preparations of Lactiplantibacillus plantarum CNCM I-3235, Lactiplantibacillus plantarum DSM 11672/CNCM I-3736, Pediococcus acidilactici CNCM I-3237, Pediococcus acidilactici DSM 11673/CNCM I-4622, Pediococcus pentosaceus NCIMB 12455, Acidipropionibacterium acidipropionici CNCM I-4661, Lentilactobacillus buchneri NCIMB 40788/CNCM I-4323 and Lentilactobacillus hilgardii CNCM I-4785 and Lentilactobacillus buchneri CNCM I-4323/NCIMB 40788 as feed additives for all animal species
2024/252 concerning the renewal of the authorisation of a preparation of Lactiplantibacillus plantarum DSM 23375 as a feed additive for all animal species
2024/260 concerning the authorisation of cumin essential oil from Cuminum cyminum L., sweet fennel tincture from Foeniculum vulgare Mill. ssp. vulgare var. dulce, dong quai tincture from Angelica sinensis (Oliv.) Diels, parsley tincture from Petroselinum crispum (Mill.) Fuss, star anise tincture from Illicium verum Hook f., asafoetida essential oil from Ferula-assa-foetida L., dill herb essential oil from Anethum graveolens L. and dill tincture from Anethum graveolens L. as feed additives for certain animal species
2024/261 concerning the authorisation of black pepper essential oil and black pepper oleoresin from Piper nigrum L. as feed additives for all animal species and black pepper supercritical extract from Piper nigrum L. as a feed additive for cats and dogs
2024/262 concerning the renewal of the authorisation of a preparation of endo-1,4-beta-glucanase produced by Trichoderma citrinoviride IMI 360748 as a feed additive for chickens for fattening, minor poultry species for fattening and weaned piglets, the authorisation of that preparation as a feed additive for turkeys for fattening, all poultry species reared for laying or breeding, ornamental birds and suckling piglets
2024/285 concerning the authorisation of taiga root tincture from Eleutherococcus senticosus (Rupr. & Maxim.) Maxim as a feed additive for dogs, cats and horses
2024/749 concerning the authorisation of lignosulphonate as a feed additive for all animal species
2024/750 concerning the renewal of the authorisation of thaumatin as a feed additive for all animal species
2024/752 concerning the denial of authorisation of a preparation of astaxanthin-rich Phaffia rhodozyma (ATCC SD-5340) as a feed additive for salmon and trout
2024/754 amending Implementing Regulation (EU) 2020/1762 as regards the terms of the authorisation of a preparation of Bacillus subtilis DSM 32324, Bacillus subtilis DSM 32325 and Bacillus amyloliquefaciens DSM 25840 as a feed additive for all poultry species for fattening or reared for laying or reared for breeding
2024/763 concerning the renewal of the authorisation of preparations of Lactiplantibacillus plantarum NCIMB 30083 and Lactiplantibacillus plantarum NCIMB 30084 as feed additives for all animal species
Tables & Figures
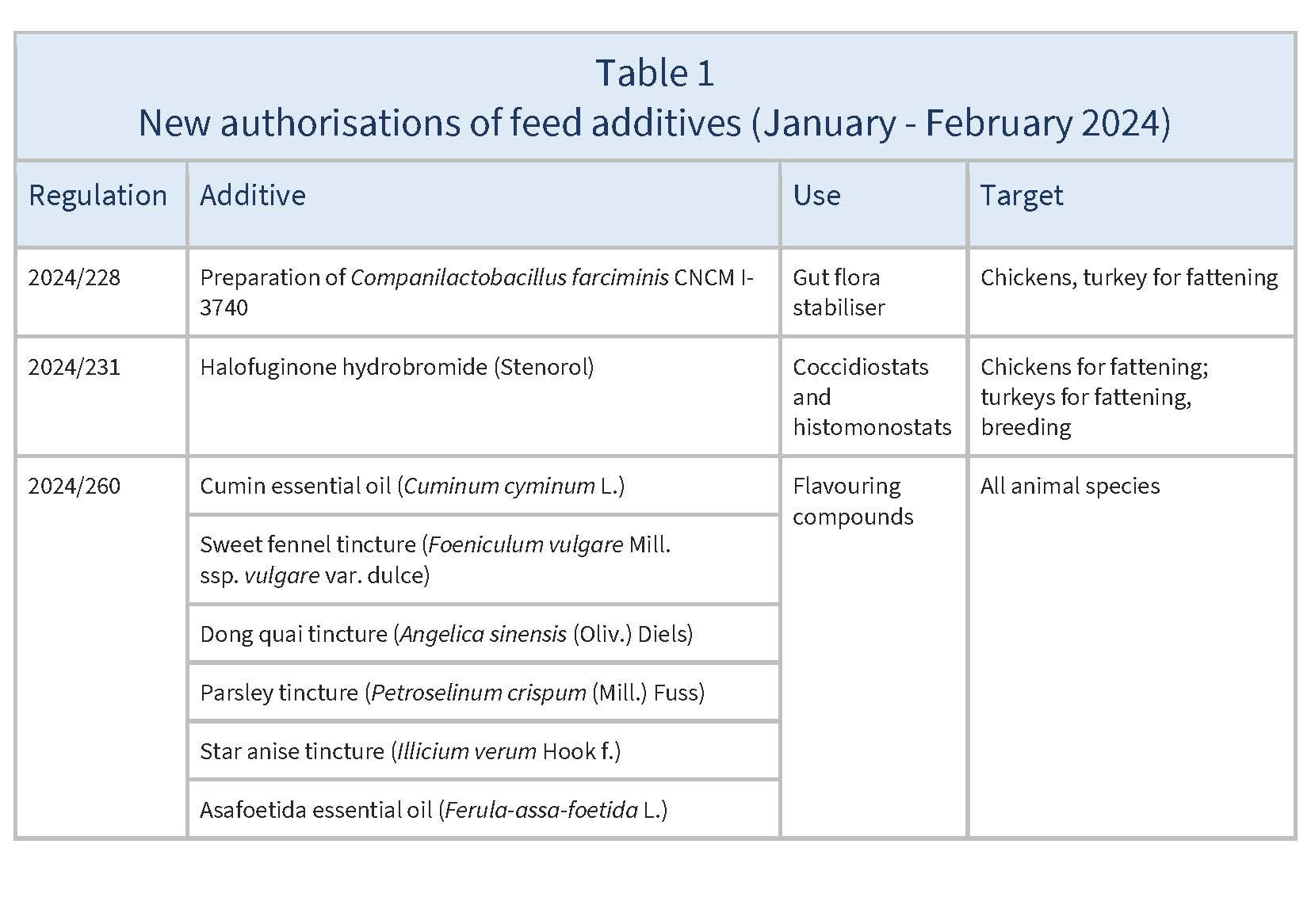
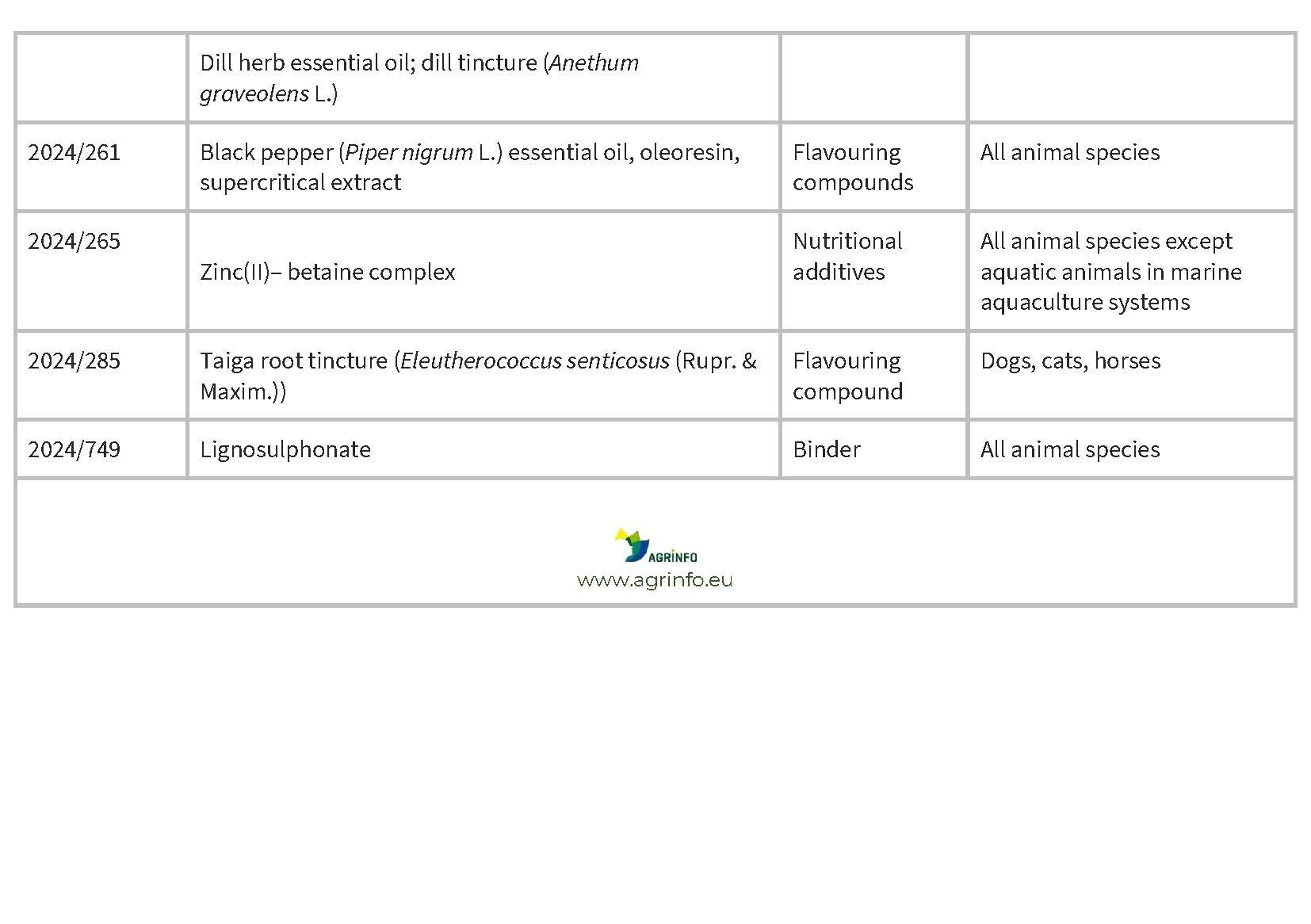
Source: based on Regulations 2024/228, 2024/231, 2024/260, 2024/261, 2024/265, 2024/285, 2024/749
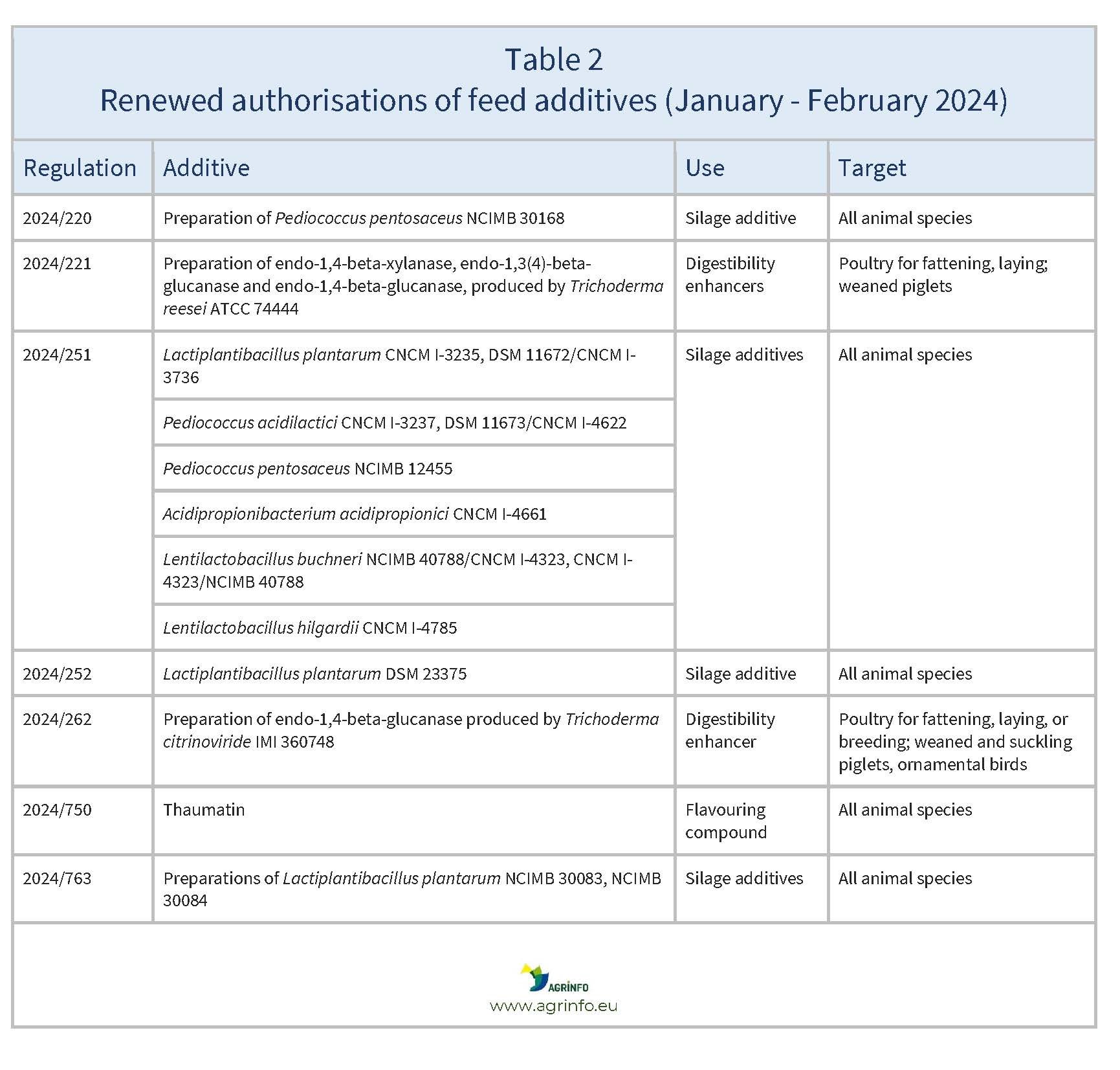
Source: based on Regulations 2024/220, 2024/221, 2024/251, 2024/252, 2024/262, 2024/750, 2024/763
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU authorises or reauthorises certain feed additives, denies other authorisations
Commission Implementing Regulations 2024/220, 2024/221, 2024/228, 2024/231, 2024/239, 2024/251, 2024/252, 2024/260, 2024/261, 2024/262, 2024/265, 2024/285, 2024/749, 2024/750, 2024/752, 2024/754, 2024/763
What is changing and why?
In January and February 2024, the EU authorised the new feed additives listed in Table 1. Applications for these authorisations were submitted and considered by the Reference Laboratory set up by the Feed Additives Regulation (1831/2003).
The EU reauthorised the feed additives listed in Table 2, but denied authorisation of a preparation of astaxanthin-rich Phaffia rhodozyma (ATCC SD-5340) as a feed additive for salmon and trout, because the European Food Safety Authority (EFSA) could not conclude on its safety for consumers.
The EU authorises the use for poultry of certain Bacillus preparations together with certain coccidiostats because EFSA considers that these uses are compatible.
Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of feed additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel format (see foot of Food and Feed Information Portal).
Timeline
Regulations 2024/220, 2024/221, 2024/228, 2024/231, 2024/260 apply from 1 February 2024. These authorisations remain valid until 4 February 2034.
Regulations 2024/251, 2024/252 and 2024/285 apply from 6 February 2024. These authorisations remain valid until 6 February 2034.
Regulations 2024/261, 2024/262, 2024/265 and 2024/285 apply from 7 February 2024. These authorisations remain valid until 7 February 2034.
Regulations 2024/749, 2024/750, 2024/752, 2024/754 and 2024/763 will apply from 21 March 2024. These authorisations remain valid until 21 March 2034.
Tables & Figures


Source: based on Regulations 2024/228, 2024/231, 2024/260, 2024/261, 2024/265, 2024/285, 2024/749

Source: based on Regulations 2024/220, 2024/221, 2024/251, 2024/252, 2024/262, 2024/750, 2024/763
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
