Feed additives: August–September 2025 authorisations
- Feed additives
- Feed safety
Summary
Overview of the latest authorisations of feed additives and their use in animal nutrition in target animals.
Overview of recent feed additive authorisations
Commission Implementing Regulations 2025/1782, 2025/1784, 2025/1787, 2025/1795, 2025/1915, 2025/1928
Update
Overview of the latest authorisations of feed additives and their use in animal nutrition in target animals.
Impacted Products
Prepared fodder
Authorisations
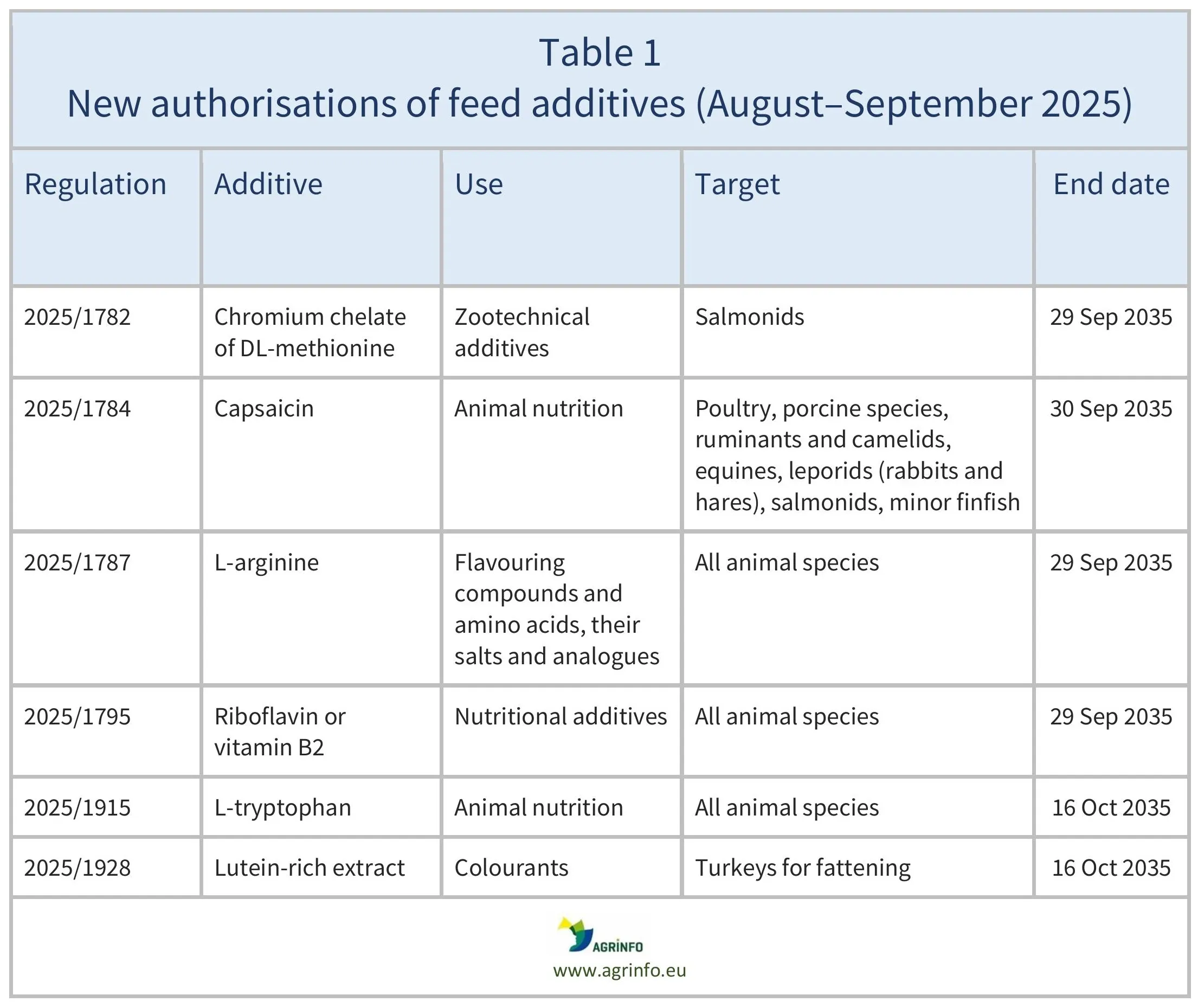
In August–September 2025, the European Union (EU) authorised the feed additives listed in Table 1, based on opinions published by the European Food Safety Authority (EFSA; see Resources). The conditions of use are described in the respective Regulations.
Applications for the above authorisations were submitted and considered by the Reference Laboratory set up by the Feed Additives Regulation (1831/2003).
Timeline
The authorisations remain valid until the end dates listed in Table 1.
What are the major implications for exporting countries?
With these authorisations, more feed additives will be available on the market. Authorisations and renewals are valid for 10 years. The use of all preparations and substances specified as feed additives must comply with the provisions of use specified in the Annex to each Regulation.
Recommended Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel (see Food and Feed Information Portal: Feed Additives > Download Register in Excel format).
Background
The procedure for authorising the placing on the market and use of feed additives is set out in Regulation 1831/2003.
Resources
EU Feed Additives register
Regulation 1831/2003 on additives for use in animal nutrition
EFSA (2020a) Safety and efficacy of Availa®Cr chromium chelate of DL-methionine) as a feed additive for dairy cows. EFSA Journal, 18(2): e06026.
EFSA (2020b) Safety and efficacy of a feed additive consisting of lutein-rich extract of Tagetes erecta L. for turkeys for fattening (EW Nutrition) EFSA Journal, 22(10): e9027.
EFSA (2025a) Safety and efficacy of feed additives consisting of vitamin B2 (98%) and vitamin B2 (80%) produced with Bacillus subtilis CGMCC 7.449 for all animal species (Chifeng Pharmaceutical Co., Ltd.). EFSA Journal, 23(2): e9249.
EFSA (2025b) Safety and efficacy of a feed additive consisting of L-arginine produced with Corynebacterium glutamicum KCCM 80387 for all animal species (CJ Europe GmbH). EFSA Journal, 23(2): e9528.
EFSA (2025c) Safety and efficacy of a feed additive consisting of chromium chelate of DL-methionine (Availa® Cr) for salmonids (Zinpro Animal Nutrition Europe, Inc.). EFSA Journal, 23(3): e9310.
EFSA (2025d) Safety and efficacy of a feed additive consisting of L-tryptophan produced with Corynebacterium glutamicum KCCM 80346 for all animal species (CJ Europe GmbH). EFSA Journal, 23(4): e9327.
EFSA (2025e) Safety and efficacy of a feed additive consisting of capsaicin for all animal species (XP Chemistries AB). EFSA Journal, 23(4): e9340.
Sources
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
Overview of recent feed additive authorisations
Commission Implementing Regulations 2025/1782, 2025/1784, 2025/1787, 2025/1795, 2025/1915, 2025/1928
Authorisations
In August–September 2025, the European Union (EU) authorised the feed additives listed in Table 1, based on opinions published by the European Food Safety Authority. The conditions of use are described in the respective Regulations.
Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel (see Food and Feed Information Portal: Feed Additives > Download Register in Excel format).
Timeline
The authorisations remain valid until the end dates listed in Table 1.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.