Feed additives: Authorisations and withdrawals May–June 2024
- Feed additives
- Feed safety
Summary
An overview of the latest authorisations of feed additives, and their use in animal nutrition in target animals, and withdrawals, May–June 2024.
EU authorises certain feed additives and withdraws others
Commission Implementing Regulations 2024/1325, 2024/1685, 2024/1723, 2024/1727, 2024/1730, 2024/1743, 2024/1750, 2024/1755, 2024/1757, 2024/1786
Update
An overview of the latest authorisations of feed additives, and their use in animal nutrition in target animals, and withdrawals, May–June 2024.
Impacted Products
Feed additives, prepared fodder
What is changing?
New authorisations and reauthorisations
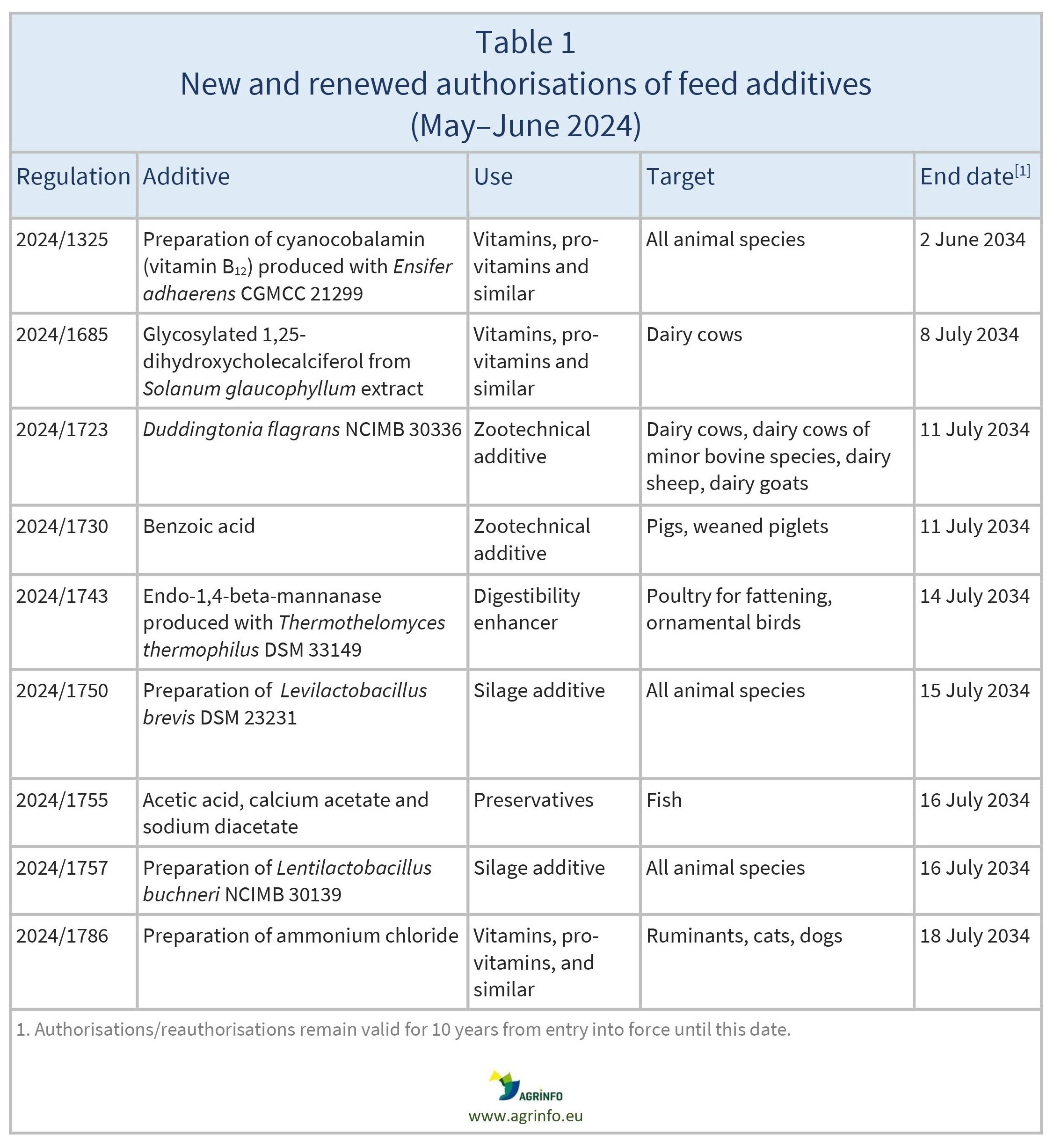
In May and June 2024, the EU authorised or reauthorised the feed additives listed in Table 1.
These authorisations are based on opinions published by the European Food Safety Authority (EFSA) [see Resources 1–10].
The conditions of use are described in the respective Regulations.
Withdrawals
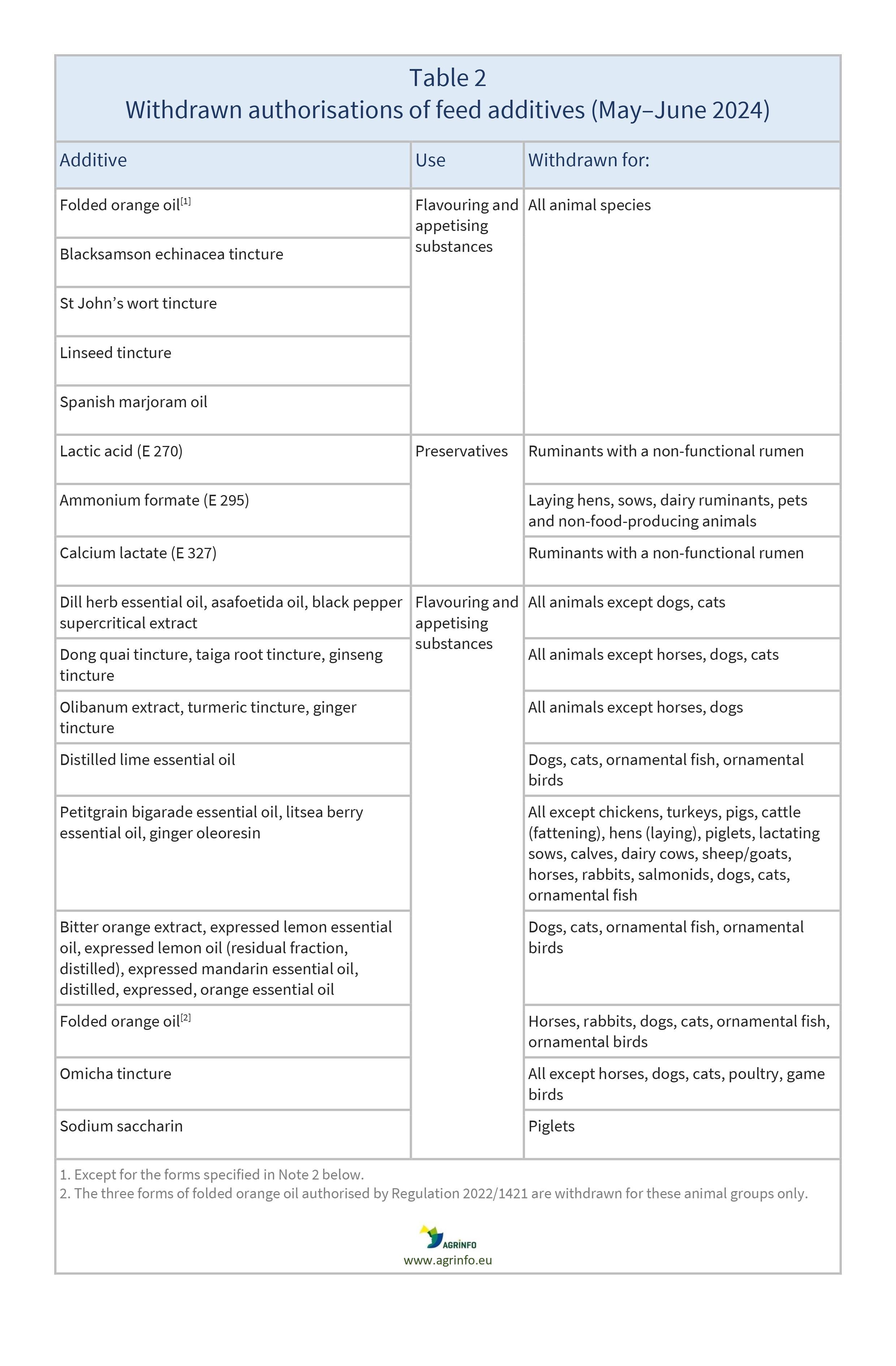
The feed additives listed in Table 2 will be withdrawn from the market (Regulation 2024/1727).
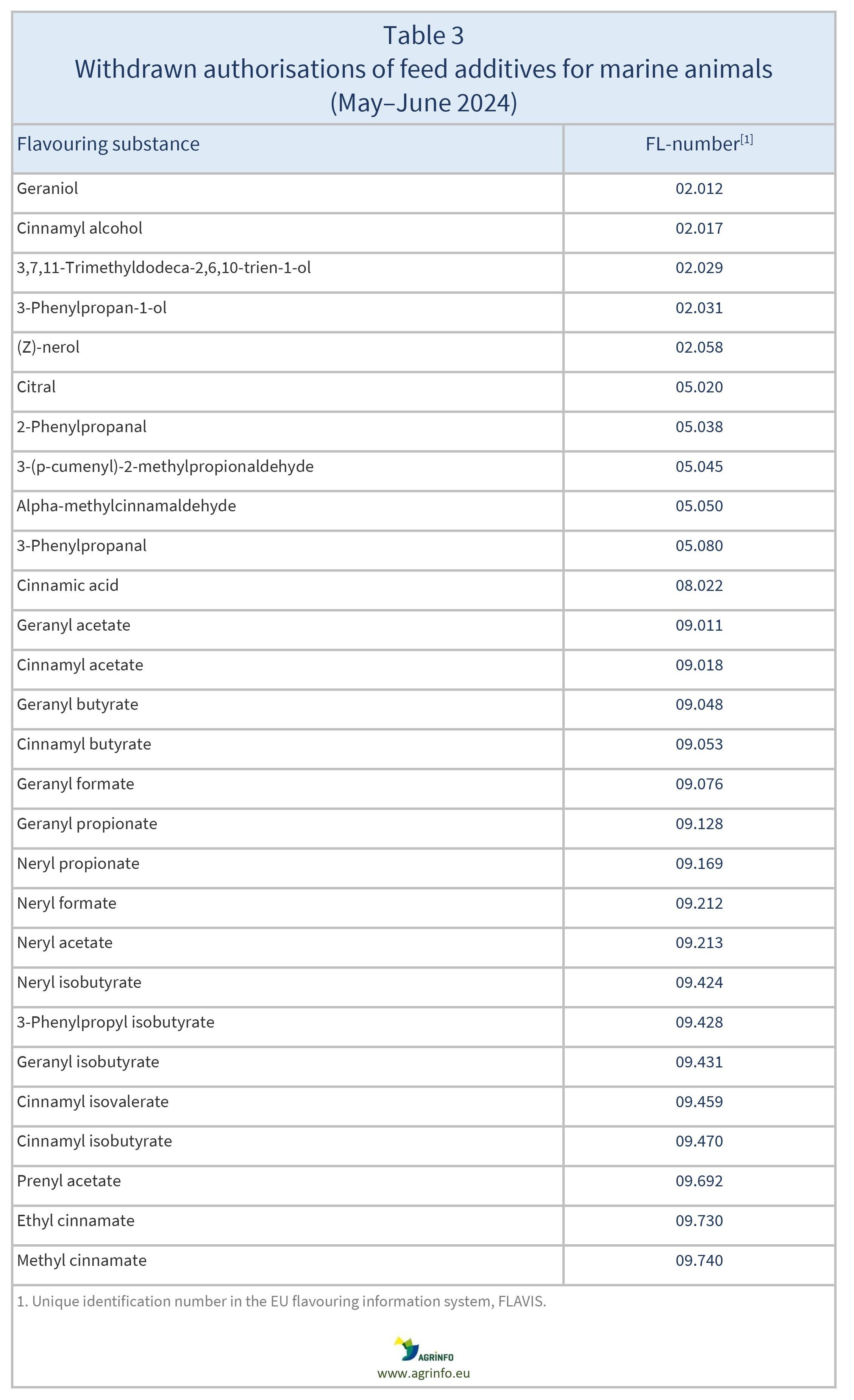
Various natural products and corresponding synthetic products used as flavouring and appetising substances in feed for marine animals are also withdrawn (see Table 3).
Why?
Applications for the above authorisations were submitted and considered by the Reference Laboratory set up by the Feed Additives Regulation (1831/2003). That Regulation also requires feed additives to be withdrawn from the market if no application has been submitted before the deadline provided, or if an application was submitted but subsequently withdrawn. In cases where applications have been submitted or withdrawn only for certain animal species or categories, the withdrawal only concerns those species and categories specified.
Timeline
The new authorisations and reauthorisations remain valid until the end dates listed in Table 1.
Regulation 2024/1727 (Withdrawals) took effect on 11 July 2024.
Transitional period:
- existing stocks, 12 months
- premixtures, 15 months
- compound feed, 24 months.
What are the major implications for exporting countries?
With these new authorisations, more feed additives will be available on the market. Authorisations and renewals are valid for 10 years. The use of all preparations and substances specified as feed additives must comply with the provisions of use specified in the Annex to each Regulation.
Recommended Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of the feed additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel format (see foot of Food and Feed Information Portal).
Background
The procedure for authorising the placing on the market and use of feed additives is set out in Regulation (EC) 1831/2003. For the latest updates on feed additives see the EU Feed Additives register.
Resources
Opinions published by the European Food Safety Authority on the safety/efficacy of the following feed additives:
- EFSA (2022) Safety and efficacy of the feed additive consisting of ammonium chloride (Ammonium Chloride AF) for all ruminants, dogs and cats for the renewal of its authorisation. EFSA Journal, 20(4): 7255.
- EFSA (2022) Safety and efficacy of a feed additive consisting of Solanum glaucophyllum leaf extract for dairy cows and other dairy ruminants. EFSA Journal, 20(8): 7434.
- EFSA (2023) Assessment of the safety of the feed additives acetic acid, calcium acetate and sodium diacetate for fish. EFSA Journal, 21(7): 8176.
- EFSA (2023) Safety and efficacy of a feed additive consisting of 25‐hydroxycholecalciferol produced with Saccharomyces cerevisiae CBS 146008 for pigs and poultry for the renewal of its authorisation. EFSA Journal, 21(8): 8168.
- EFSA (2023) Safety of a feed additive consisting of Duddingtonia flagrans NCIMB 30336 (BioWorma®) for all grazing animals. EFSA Journal, 21(11): e8465.
- EFSA (2023) Safety and efficacy of a feed additive consisting of benzoic acid (Kalama® Animal Feed Grade Benzoic acid) for weaned piglets and pigs for fattening. EFSA Journal, 21(12): e8454.
- EFSA (2023) Assessment of the feed additive consisting of Levilactobacillus brevis DSM 23231 for all animal species for the renewal of its authorization. EFSA Journal, 21(12): e8461.
- EFSA (2023) Assessment of the feed additive consisting of Lentilactobacillus buchneri (formerly Lactobacillus buchneri) NCIMB 30139 for all animal species for the renewal of its authorisation. EFSA Journal, 21(12): e8511.
- EFSA (2024) Safety of a feed additive consisting of endo 1,4 β-d-mannanase produced by Thermothelomyces thermophilus DSM 33149 (Natupulse® TS/TS L) for chickens and turkeys for fattening, minor poultry species for fattening and ornamental birds. EFSA Journal, 22(2): e8632.
- EFSA (2024) Safety and efficacy of a feed additive consisting of vitamin B12 (cyanocobalamin) produced by fermentation with Ensifer adhaerens CGMCC 21299 for all animal species. EFSA Journal, 22(4): e8752.
Resources from the European Commission:
EU Feed Additives register
Regulation 1831/2003 on additives for use in animal nutrition
Sources
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU authorises certain feed additives and withdraws others
Commission Implementing Regulations 2024/1325, 2024/1685, 2024/1723, 2024/1727, 2024/1730, 2024/1743, 2024/1750, 2024/1755, 2024/1757, 2024/1786
What is changing and why?
New authorisations and reauthorisations
In May and June 2024, the EU authorised or reauthorised the feed additives listed in Table 1.
These authorisations are based on opinions published by the European Food Safety Authority (EFSA).
The conditions of use are described in the respective Regulations.
Withdrawals
The feed additives listed in Table 2 will be withdrawn from the market (Regulation 2024/1727).
Various natural products and corresponding synthetic products used as flavouring and appetising substances in feed for marine animals are also withdrawn (see Table 3).
Timeline
The new authorisations and reauthorisations remain valid until the end dates listed in Table 1.
Regulation 2024/1727 (Withdrawals) took effect on 11 July 2024.
Transitional period:
- existing stocks, 12 months
- premixtures, 15 months
- compound feed, 24 months.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.