Feed additives: December authorisations
- Feed additives
- Feed safety
Summary
An overview of the latest authorisations of feed additives and their use in animal nutrition in target animals.
EU authorises certain feed additives
Commission Implementing Regulations 2023/2732, 2023/2733, 2023/2734, 2023/2736, 2023/2802, 2023/2846, 2023/2850
Update
An overview of the latest authorisations of feed additives and their use in animal nutrition in target animals.
Impacted Products
Feed additives, prepared fodder
New authorisations (December 2023)
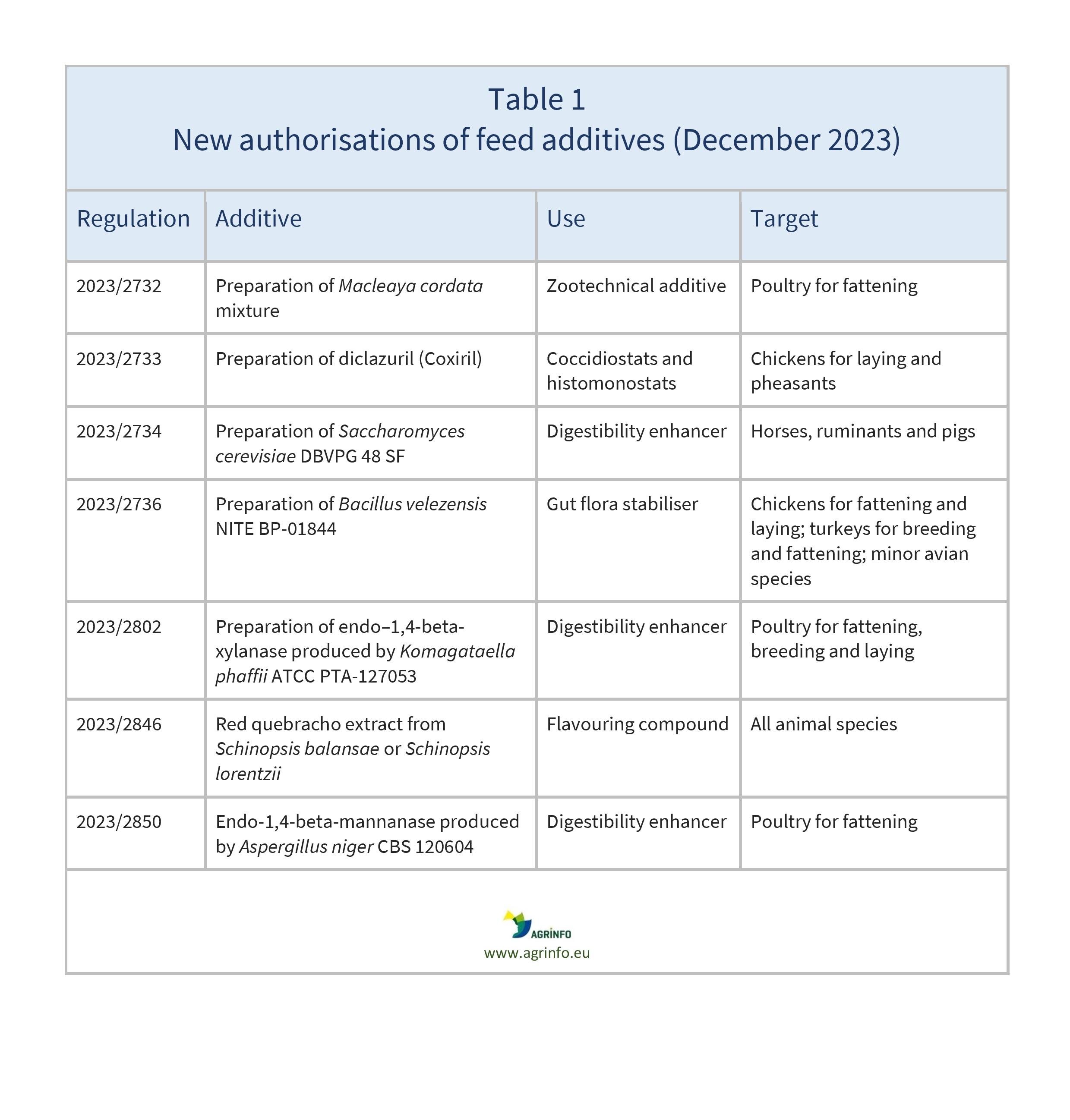
In December 2023, the EU authorised the new feed additives listed in Table 1.
These authorisations are based on the following opinions published by the European Food Safety Authority: EFSA (2022), (2023a), (2023b), (2023c), (2023d), (2023e), (2023f).
Why?
Applications for the above authorisations were submitted and considered by the Reference Laboratory set up by the Feed Additives Regulation (1831/2003).
Timeline
Regulations 2023/2732, 2023/2733, 2023/2734, and 2023/2736 apply from 28 December 2023. These authorisations remain valid until 28 December 2033.
Regulation 2023/2802 applies from 8 January 2024. This authorisation remains valid until 8 January 2034.
Regulations 2023/2846 and 2023/2850 apply from 10 January 2024. These authorisations remain valid until 10 January 2034.
What are the major implications for exporting countries?
With these new authorisations, more feed additives will be available on the market. Authorisations and renewals are valid for 10 years. The way that all preparations and substances specified as feed additives are used must comply with the provisions of use specified in the Annex to each Regulation.
Recommended Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of the feed additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel format (see foot of Food and Feed Information Portal).
Background
The procedure for authorising the placing on the market and use of feed additives is set out in Regulation (EC) 1831/2003. For the latest updates on feed additives see the EU Feed Additives register.
Resources
Online resources from the European Commission:
- Regulation 1831/2003 on additives for use in animal nutrition
- EU Feed Additives register
Reviews by the European Food Safety Authority:
- EFSA (2022) Safety and efficacy of a feed additive consisting of an extract of condensed tannins from Schinopsis balansae Engl. and Schinopsis lorentzii (Griseb.) Engl. (red quebracho extract) for use in all animal species (FEFANA asbl). EFSA Journal, 20(12): 7699.
- EFSA (2023a) Safety for the environment of a feed additive consisting of diclazuril (Coxiril®) for chickens reared for laying and pheasants (Huvepharma NV). EFSA Journal, 21(4): 7963.
- EFSA (2023b) Safety and efficacy of a feed additive consisting of Saccharomyces cerevisiae DBVPG 48 SF (BioCell®) for horses, pigs and ruminants (Mazzoleni S.p.A.). EFSA Journal, 21(4): 7971.
- EFSA (2023c) Safety and efficacy of a feed additive consisting of β‐mannanase produced by Aspergillus niger CBS 120604 (Nutrixtend Optim) for use in all poultry for fattening (Kerry Ingredients & Flavours Ltd). EFSA Journal, 21(6): 8045.
- EFSA (2023d) Efficacy of a feed additive consisting of endo‐1,4‐beta‐xylanase produced by Komagataella phaffii ATCC PTA‐127053 (Xygest™ HT) for all poultry species (Kemin Europa N.V.). EFSA Journal, 21(6): 8047.
- EFSA (2023e) Safety and efficacy of a feed additive consisting of Macleaya cordata (Willd.) R. Br. extract and leaves (Sangrovit® extra) for all poultry species (excluding laying and breeding birds) (Phytobiotics Futterzusatzstoffe GmbH). EFSA Journal, 21(6): 8052.
- EFSA (2023f) Efficacy of a feed additive consisting of Bacillus velezensis NITE BP‐01844 (BA‐KING®) for chickens for fattening, chickens reared for laying, turkeys for fattening, turkeys reared for breeding and all avian species for fattening, or rearing to slaughter or point of lay including non‐food producing species (Toa Biopharma Co., Ltd.). EFSA Journal, 21(6): 8053.
Sources
Commission Implementing Regulations:
- 2023/2732 concerning the authorisation of a preparation of Macleaya cordata mixture as a feed additive for all poultry species for fattening
- 2023/2733 concerning the authorisation of a preparation of diclazuril (Coxiril) as a feed additive for chickens reared for laying and pheasants
- 2023/2734 concerning the authorisation of a preparation of Saccharomyces cerevisiae DBVPG 48 SF as a feed additive for horses, dairy ruminants and pigs
- 2023/2736 concerning the authorisation of a preparation of Bacillus velezensis NITE BP-01844 as a feed additive for all poultry species for fattening, chickens reared for laying, turkeys reared for breeding, minor poultry species reared for laying or for breeding and ornamental birds
- 2023/2802 concerning the authorisation of a preparation of endo–1,4-beta-xylanase produced by Komagataella phaffii ATCC PTA-127053 as a feed additive for all poultry species for fattening, breeding, and reared for laying or breeding
- 2023/2846 concerning the authorisation of red quebracho extract from Schinopsis balansae or Schinopsis lorentzii as a feed additive for all animal species
- 2023/2850 concerning the authorisation of a preparation of endo-1,4-beta-mannanase produced by Aspergillus niger CBS 120604 as a feed additive for all poultry species for fattening
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU authorises certain feed additives
Commission Implementing Regulations 2023/2732, 2023/2733, 2023/2734, 2023/2736, 2023/2802, 2023/2846, 2023/2850
What is changing and why?
In December 2023 the EU authorised the new feed additives listed in Table 1. Applications for these authorisations were submitted and considered by the Reference Laboratory set up by the Feed Additives Regulation (1831/2003).
Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of feed additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel format (see foot of Food and Feed Information Portal).
Timeline
Regulations 2023/2732, 2023/2733, 2023/2734, and 2023/2736 apply from 28 December 2023. These authorisations remain valid until 28 December 2033.
Regulation 2023/2802 applies from 8 January 2024. This authorisation remains valid until 8 January 2034.
Regulations 2023/2846 and 2023/2850 apply from 10 January 2024. These authorisations remain valid until 10 January 2034.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.