Feed additives: January–February 2025 authorisations, reauthorisations, and corrections
- Feed additives
Summary
Overview of the latest authorisations and reauthorisations of feed additives and their use in animal nutrition in target animals, including corrections to certain existing authorisations.
EU authorises and reauthorises certain feed additives
Commission Implementing Regulations: 2025/142, 2025/143, 2025/148, 2025/151, 2025/152, 2025/157, 2025/159, 2025/160, 2025/161, 2025/168, 2025/169, 2025/183, 2025/188, 2025/193, 2025/272, 2025/273, 2025/275, 2025/276, 2025/277, 2025/278, 2025/279, 2025/281, 2025/284, 2025/314, 2025/316, 2025/353, 2025/359, 2025/364
Corrections: 2025/181, 2025/182, 2025/187
Update
Overview of the latest authorisations and reauthorisations of feed additives and their use in animal nutrition in target animals, including corrections to certain existing authorisations.
Impacted Products
Feed additives, prepared fodder
What is changing?
Authorisations
In January–February 2025, the European Union (EU) authorised the feed additives listed in Table 1, based on opinions published by the European Food Safety Authority (EFSA) (see Resources 2, 11–13, 16, 18, 22, 29–31). The conditions of use are described in the respective Regulations.
Reauthorisations
In January–February 2025, the EU reauthorised the feed additives listed in Table 2, based on opinions published by EFSA (see Resources 3–8, 10, 14–15, 17, 19–21, 23–28, 32). The conditions of use are described in the respective Regulations.
Amended and corrected authorisations
Regulation 2025/183 increases the recommended maximum content of a feed additive consisting of nonanoic acid from 5 to 100 mg/kg complete feed for all poultry for fattening, laying, or breeding, and all Suidae for fattening, including suckling and weaned piglets.
Regulation 2025/316 changes the name of the holder of the authorisations for a preparation of 6-phytase produced by Trichoderma reesei CBS 122001 and a preparation of endo-1,4-beta-xylanase produced by T. reesei CBS 114044.
Regulation 2025/181 corrects Regulation 2024/2393 regarding the animal categories for which the use of sodium bisulphate remains authorised until 10 September 2025.
Regulation 2025/182 corrects Regulation 2019/901 regarding the chemical formula of riboflavin 5′-phosphate monosodium salt.
Regulation 2025/187 corrects Regulation 2022/415 regarding the use of acetic acid, sodium diacetate, and calcium acetate as feed additives for all animal species other than fish. The safety of use of these three additives without a need to establish a maximum content was demonstrated only for ruminants (EFSA 2021).
Why?
Applications for the above authorisations and reauthorisations were submitted and considered by the Reference Laboratory set up by the Feed Additives Regulation (1831/2003).
The recommended maximum content of the feed additive consisting of nonanoic acid was increased because EFSA (2024) [Resource 9] concluded that its use is safe at 100 mg/kg feed for poultry and Suidae.
Timeline
The authorisations and reauthorisations remain valid until the end dates listed in Tables 1 and 2.
What are the major implications for exporting countries?
With these authorisations, more feed additives will be available on the market. Authorisations and renewals are valid for 10 years. The use of all preparations and substances specified as feed additives must comply with the provisions of use specified in the Annex to each Regulation.
Recommended Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of the feed additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel format (see foot of Food and Feed Information Portal webpage).
Background
The procedure for authorising the placing on the market and use of feed additives is set out in Regulation 1831/2003. For the latest updates on feed additives see the EU Feed Additives register.
Resources
EU Feed Additives register
Regulation 1831/2003 on additives for use in animal nutrition
Regulation 2024/2393 concerning the renewal of the authorisation of sodium bisulphate and the authorisation of new uses of that substance as a feed additive for certain animal species
- EFSA (2021) Safety and efficacy of a feed additive consisting of acetic acid for all animal species. EFSA Journal, 19(6): 6615.
- EFSA (2023) Safety and efficacy of a feed additive consisting of Saccharomyces cerevisiae DBVPG 48 SF (BioCell®) for horses, pigs and ruminants (Mazzoleni S.p.A.). EFSA Journal, 21(4): 7971.
- EFSA (2023) Assessment of a feed additive containing Enterococcus lactis NCIMB 11181 (Lactiferm®) for weaned piglets, calves for fattening and calves for rearing for the renewal of its authorisation (Chr. Hansen A/S) EFSA Journal, 21: e8466.
- EFSA (2024) Safety of feed additives consisting of microcrystalline cellulose and carboxymethyl cellulose for all animal species (International Cellulosics Association). EFSA Journal, 22: e8625.
- EFSA (2024) Safety of a feed additive consisting of hydroxypropyl cellulose for all animal species (International Cellulosics Association). EFSA Journal, 22: e8626.
- EFSA (2024) Safety of a feed additive consisting of ethyl cellulose for all animal species (International Cellulosics Association). EFSA Journal, 22: e8636.
- EFSA (2024) Safety of feed additives consisting of hydroxypropyl methyl cellulose (E 464) and methyl cellulose (E 461) for all animal species (International Cellulosics Association) EFSA Journal, 22: e8637.
- EFSA (2024) Safety of a feed additive consisting of propyl gallate for all animal species (FEFANA asbl). EFSA Journal, 22: e8638.
- EFSA (2024) Modification of the terms of authorisation regarding the maximum inclusion level of a feed additive consisting of nonanoic acid for all pigs and poultry species (Anitox Corporation). EFSA Journal, 22(2): e8642.
- EFSA (2024) Assessment of the feed additive consisting of Pediococcus pentosaceus DSM 14021 for all animal species for the renewal of its authorisation (Chr. Hansen A/S). EFSA Journal, 22(4): e8706.
- EFSA (2024) Safety and efficacy of a feed additive consisting of l-tryptophan (produced with Escherichia coli CGMCC 7.460) for all animal species (Kempex Holland B.V.). EFSA Journal, 22(4): e8707.
- EFSA (2024) Safety and efficacy of a feed additive consisting of l‐threonine (produced with Escherichia coli CGMCC 7.455) for all animal species (Kempex Holland B.V.). EFSA Journal, 22(4): e8708.
- EFSA (2024) Safety and efficacy of a feed additive consisting of 6-phytase produced with Trichoderma reesei (CBS 126897) (Quantum® Blue) for fin fish (ROAL Oy). EFSA Journal, 22: e8709.
- EFSA (2024) Assessment of the feed additive consisting of Saccharomyces cerevisiae MUCL 39885 (Biosprint®) for cattle for fattening for the renewal of its authorisation (Prosol SPA). EFSA Journal, 22: e8720.
- EFSA (2024) Assessment of the feed additive consisting of Levilactobacillus brevis DSM 21982 for all animal species for the renewal of its authorisation (Marigot Ltd T/A Celtic Sea Minerals). EFSA Journal, 22(4): e8725.
- EFSA (2024) Safety and efficacy of a feed additive consisting of l-isoleucine produced with Corynebacterium glutamicum CGMCC 20437 for all animal species (Eppen Europe SAS). EFSA Journal, 22(4): e8726.
- EFSA (2024) Efficacy of the feed additive consisting of Saccharomyces cerevisiae CNCM I-4407 (Actisaf® Sc47) for cattle for fattening (Lesaffre International). EFSA Journal, 22: e8727.
- EFSA (2024) Efficacy of a feed additive consisting of Saccharomyces cerevisiae DBVPG 48 SF (BioCell®) for ruminants (Mazzoleni S.p.A.). EFSA Journal, 22: e8728.
- EFSA (2024) Safety and efficacy of a feed additive consisting of a tincture derived from the roots of Panax ginseng C.A.Mey. (ginseng tincture) for horses, dogs and cats (FEFANA asbl). EFSA Journal, 22: e8730.
- EFSA (2024) Safety and efficacy of a feed additive consisting of a tincture derived from the dried fruit of Schisandra chinensis (Turcz.) Baill. (omicha tincture) for poultry, horses, dogs and cats (FEFANA asbl). EFSA Journal, 22: e8731.
- EFSA (2024) Safety and efficacy of a feed additive consisting of an essential oil derived from fresh leaves of Melaleuca cajuputi Maton & Sm. ex R. Powell and Melaleuca leucadendra (L.) L. (cajuput oil) for use in all animal species (FEFANA asbl). EFSA Journal, 22(4): 8732.
- EFSA (2024) Safety and efficacy of a feed additive consisting of muramidase produced with Trichoderma reesei DSM 32338 (Balancius™) for laying hens (DSM nutritional products). EFSA Journal, 22: e8788.
- EFSA (2024) Safety and efficacy of a feed additive consisting of a tincture derived from the flowers of Syzygium aromaticum (L.) Merr. & L.M. Perry (clove tincture) for all animal species (FEFANA asbl). EFSA Journal, 22(5):8791.
- EFSA (2024) Assessment of the feed additive consisting of Limosilactobacillus fermentum NCIMB 30169 for all animal species for the renewal of its authorisation (Microferm Ltd.). EFSA Journal, 22(5): e8794.
- EFSA (2024) Safety of the feed additive consisting of endo‐1,4‐β‐xylanase (produced with Trichoderma reesei CBS 143953), subtilisin (produced with Bacillus subtilis CBS 143946) and α‐amylase (produced with Bacillus amyloliquefaciens CBS 143954) (Avizyme® 1505) for all poultry species (Danisco (UK) Ltd.). EFSA Journal, 22: e8797.
- EFSA (2024) Safety and efficacy of a feed additive consisting of an essential oil obtained from the wood of Juniperus deppeana Steud. (cedarwood Texas oil) for use in all animal species (FEFANA asbl). EFSA Journal, 22(5): e8799.
- EFSA (2024) Assessment of the feed additive consisting of l-cystine for all animal species for the renewal of its authorisation (Bretagne Chimie Fine [BCF Life Sciences]). EFSA Journal, 22: e8800.
- EFSA (2024) Assessment of the feed additive consisting of endo-1,4-beta-xylanase (produced with Trichoderma reesei MUCL 49755), endo-1,3(4)-beta-glucanase (produced with T. reesei MUCL 49754) and polygalacturonase (produced with Aspergillus fijiensis CBS 589.94) (AveMix® 02 CS) for weaned piglets for the renewal of its authorisation and for its extension of use to suckling piglets (AVEVE BV). EFSA Journal, 22: e8854.
- EFSA (2024) Safety and efficacy of a feed additive consisting of Lactococcus lactis DSM 34262 as a silage additive for all animal species (Lactosan GmbH & Co.KG). EFSA Journal, 22: e8902.
- EFSA (2024) Safety and efficacy of a feed additive consisting of Lactiplantibacillus plantarum DSM 34271 as a silage additive for all animal species (Lactosan GmbH & Co.KG). EFSA Journal, 22: e8903.
- EFSA (2024) Safety and efficacy of a feed additive consisting of Loigolactobacillus coryniformis DSM 34345 as a silage additive for all animal species (Lactosan GmbH & Co.KG). EFSA Journal, 22: e8904.
- EFSA (2024) Assessment of the feed additive consisting of Saccharomyces cerevisiae CNCM I-4407 (Actisaf® Sc 47) for rabbits for fattening and non-food producing rabbits for the renewal of its authorisation (S. I. Lesaffre). EFSA Journal, 22: e8910.
- EFSA (2024) Assessment of the feed additive consisting of Levilactobacillus brevis DSM 16680 for all animal species for the renewal of its authorisation (Microferm Ltd.). EFSA Journal, 22(8): e8934.
Sources
Commission Implementing Regulations 2025/142, 2025/143, 2025/148, 2025/151, 2025/152, 2025/157, 2025/159, 2025/160, 2025/161, 2025/168, 2025/169, 2025/181, 2025/182, 2025/183, 2025/187, 2025/188, 2025/193, 2025/272, 2025/273, 2025/275, 2025/276, 2025/277, 2025/278, 2025/279, 2025/281, 2025/284, 2025/314, 2025/316, 2025/353, 2025/359, 2025/364
Tables & Figures
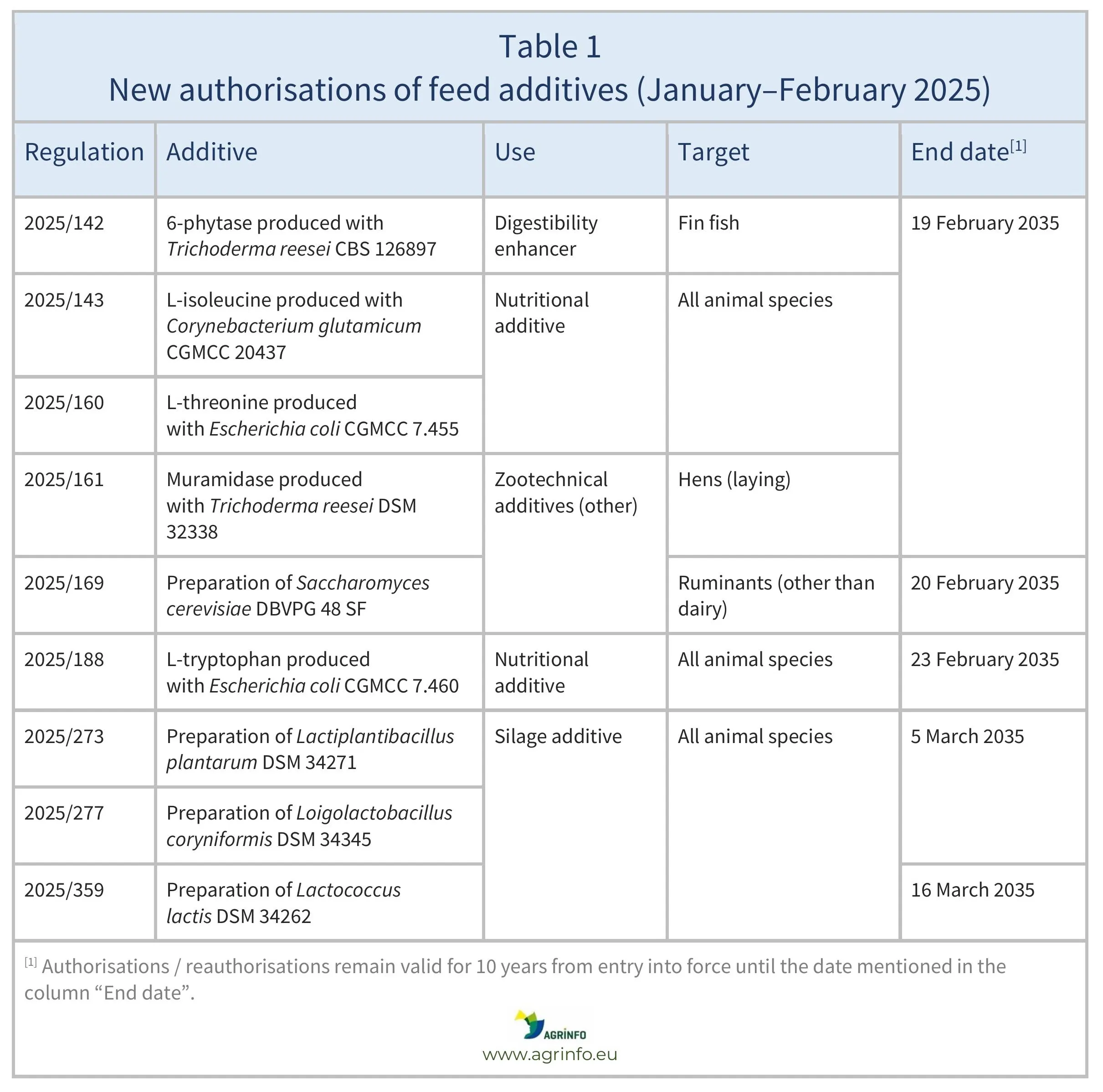
Source: based on Regulations 2025/142, 2025/143, 2025/160, 2025/161, 2025/169, 2025/188, 2025/273, 2025/277, 2025/359
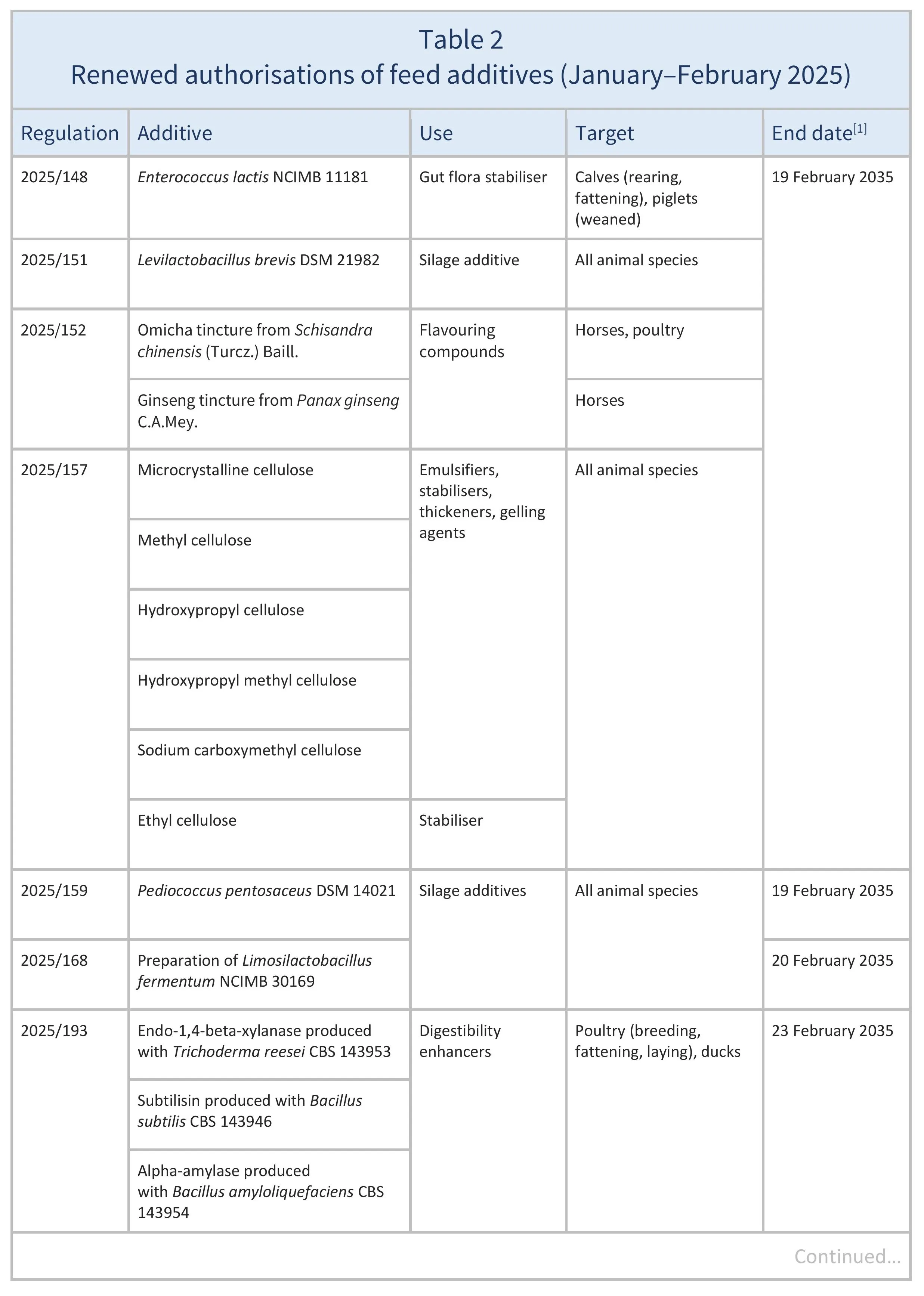
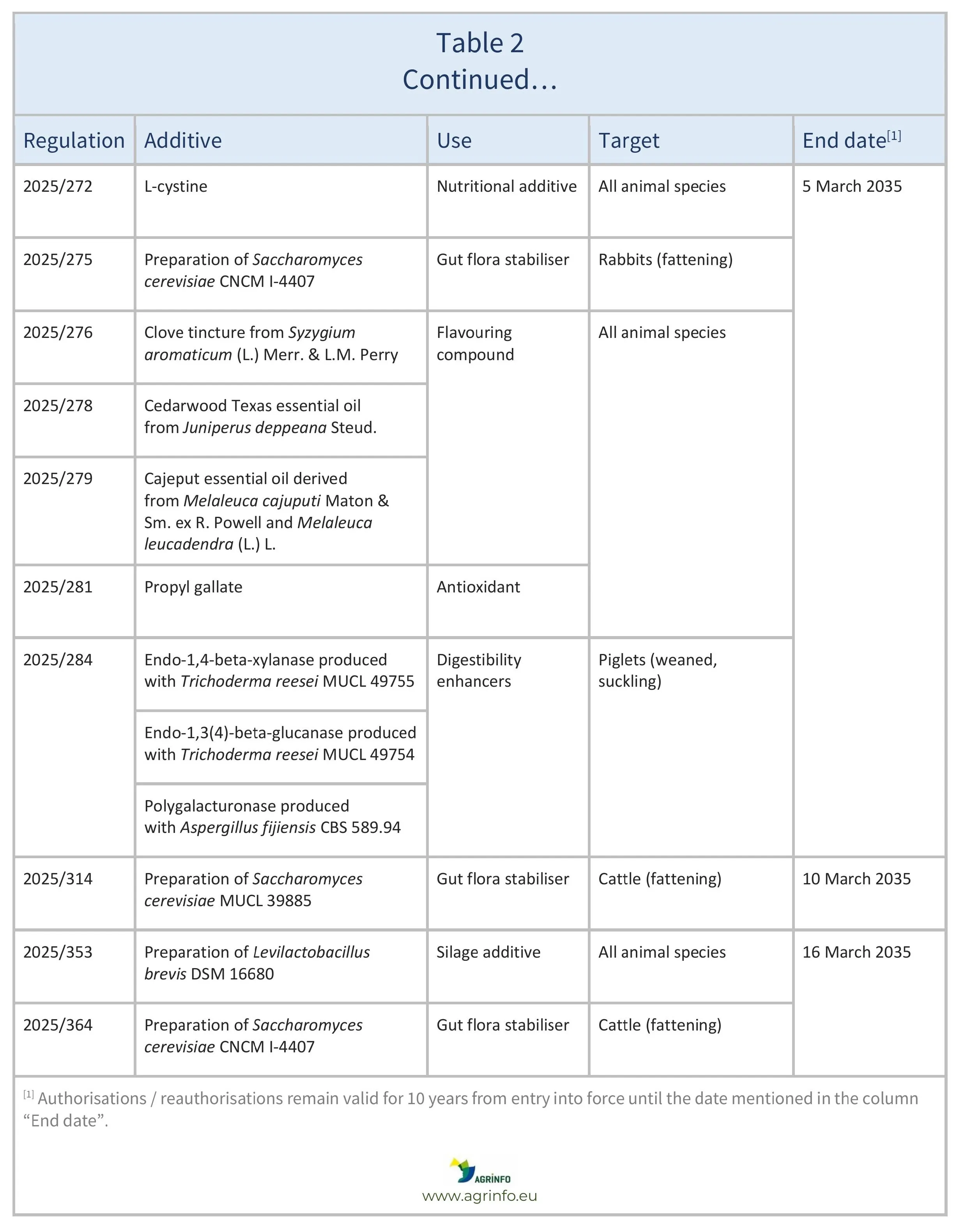
Source: based on Regulations 2025/148, 2025/151, 2025/152, 2025/157, 2025/159, 2025/168, 2025/193, 2025/272,2025/275, 2025/276, 2025/278, 2025/279, 2025/281, 2025/284, 2025/314, 2025/353, 2025/364
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU authorises and reauthorises certain feed additives
Commission Implementing Regulations 2025/142, 2025/143, 2025/148, 2025/151, 2025/152, 2025/157, 2025/159, 2025/160, 2025/161, 2025/168, 2025/169, 2025/181, 2025/182, 2025/183, 2025/187, 2025/188, 2025/193, 2025/272, 2025/273, 2025/275, 2025/276, 2025/277, 2025/278, 2025/279, 2025/281, 2025/284, 2025/314, 2025/316, 2025/353, 2025/359, 2025/364
What is changing and why?
In January and February 2025, the EU authorised or reauthorised feed additives listed in Tables 1 and 2. These authorisations are based on opinions published by the European Food Safety Authority (EFSA). The conditions of use are described in the respective Regulations.
Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of the feed additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel format (see foot of Food and Feed Information Portal webpage).
Timeline
The authorisations and reauthorisations remain valid until the end dates listed in Tables 1 and 2.
Tables & Figures

Source: based on Regulations 2025/142, 2025/143, 2025/160, 2025/161, 2025/169, 2025/188, 2025/273, 2025/277, 2025/359


Source: based on Regulations 2025/148, 2025/151, 2025/152, 2025/157, 2025/159, 2025/168, 2025/193, 2025/272,2025/275, 2025/276, 2025/278, 2025/279, 2025/281, 2025/284, 2025/314, 2025/353, 2025/364
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
