Feed additives: May–July 2025 authorisations, reauthorisations, withdrawals, and changes
- Feed additives
- Feed safety
Summary
Overview of the latest authorisations of feed additives and their use in animal nutrition in target animals.
EU authorises/reauthorises certain feed additives and withdraws others
Commission Implementing Regulations 2025/757, 2025/1254, 2025/1386, 2025/1390, 2025/1391, 2025/1392, 2025/1395, 2025/1400, 2025/1402, 2025/1403, 2025/1417, 2025/1418, 2025/1419, 2025/1423, 2025/1424, 2025/1426, 2025/1465, 2025/1468, 2025/1504, 2025/1523, 2025/1527
Notifications G/SPS/N/EU/857, G/SPS/N/EU/858, G/SPS/N/EU/863
Update
Overview of the latest authorisations of feed additives and their use in animal nutrition in target animals.
What is changing?
Authorisations
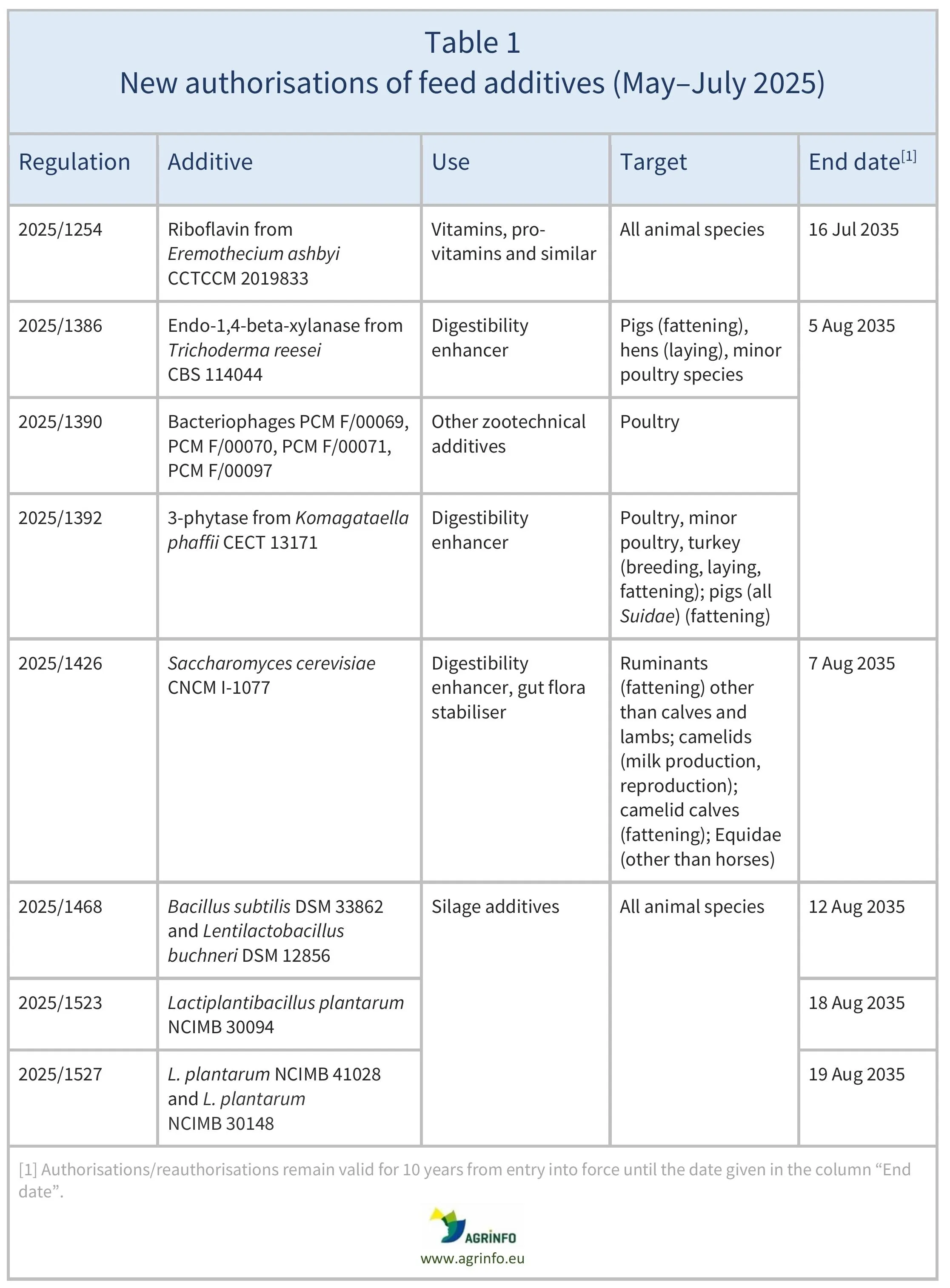
In May–July 2025, the European Union (EU) authorised the feed additives listed in Table 1, based on opinions published by the European Food Safety Authority (EFSA; see Resources). The conditions of use are described in the respective Regulations.
Reauthorisations
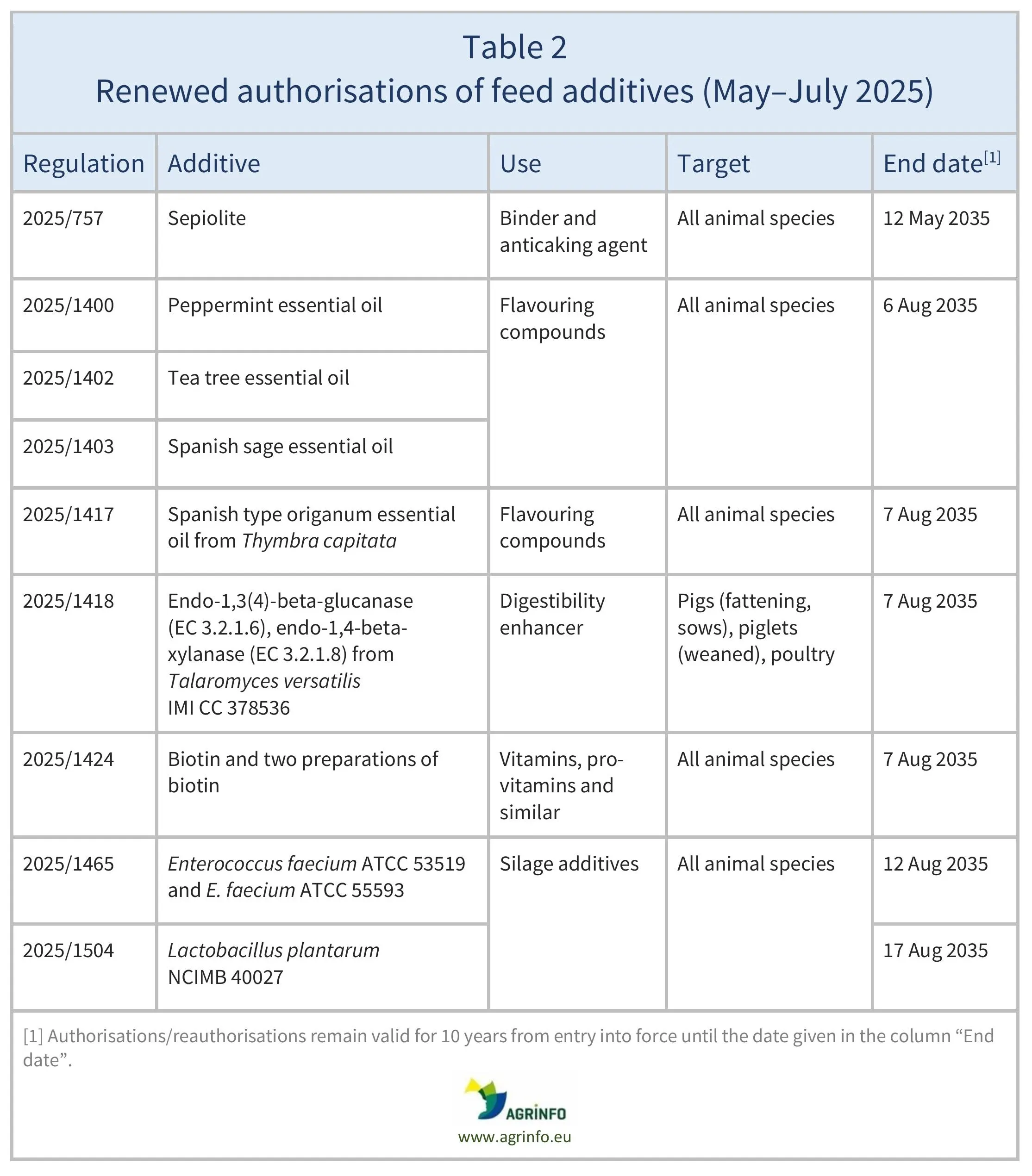
In May–July 2025, the EU renewed authorisations for the feed additives listed in Table 2, based on opinions published by EFSA (see Resources). The conditions of use are described in the respective Regulations.
The European Commission has informed the World Trade Organization Sanitary and Phytosanitary Measures (WTO SPS) Committee that it intends to renew the authorisation for eucalyptus tincture from Eucalyptus globulus as a flavouring compound in feed intended for food-producing animals listed in the draft Annex to G/SPS/N/EU/857. It also intends to renew the authorisation for celery seed essential oil for the food-producing animals listed in the draft Annex to G/SPS/N/EU/858.
Corrections and amendments
- Regulation 2025/1391 corrects Regulation 2024/1186 regarding the methyleugenol concentration in cinnamon bark essential oil, which should not exceed 0.004%.
- Regulation 2025/1395 amends and corrects Regulation 2020/997 regarding the feed additive L-lysine base (liquid) produced with Corynebacterium glutamicum NRRL B-67439 or with C. glutamicum NRRL B-67535 for all animal species (authorised until 30 July 2030).
- Regulation 2025/1419 amends Regulations 2020/148, 2021/2094, and 2023/1172 regarding a name change of authorisation holder for the feed additives decoquinate (Deccox and Avi-Deccox 60G), robenidine hydrochloride (Robenz 66G), and a preparation of lasalocid A sodium (Avatec 150 G).
- Regulation 2025/1423 amends Regulation 684/2014 regarding the preparation of canthaxanthin produced by fermentation with Yarrowia lipolytica CBS 146148 as a feed additive for breeder hens.
- Regulation 2025/1426 amends Regulation 2020/149 by modifying the authorisation of a preparation of Saccharomyces cerevisiae CNCM I-1077 as a feed additive for lambs.
Withdrawals
The European Commission has informed the WTO SPS Committee that it intends to withdraw eucalyptus tincture and celery seed essential oil as flavouring compounds in feed for certain animal species or categories. It intends to withdraw a range of other feed additives listed in the draft Annex to G/SPS/N/EU/863, either for all animal species and categories (Part 1) or for specific (Part 2) species and categories.
For feed additives that were previously authorised without a time limit but now are withdrawn from the market, a transitional period applies.
Why?
Applications for the above authorisations were submitted and considered by the Reference Laboratory set up by the Feed Additives Regulation (1831/2003). That Regulation also requires feed additives to be withdrawn from the market if no application has been submitted before the deadline provided, or if an application was submitted but subsequently withdrawn. In cases where applications have been submitted or withdrawn only for certain animal species or categories, the withdrawal only concerns those species and categories specified.
Timeline
The authorisations remain valid until the end dates listed in Tables 1 and 2.
Transitional period for withdrawals:
- existing stocks, 12 months
- premixtures, 15 months
- compound feed, 24 months.
What are the major implications for exporting countries?
With these authorisations, more feed additives will be available on the market. Authorisations and renewals are valid for 10 years. The use of all preparations and substances specified as feed additives must comply with the provisions of use specified in the Annex to each Regulation.
Recommended Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel (see Food and Feed Information Portal: Feed Additives > Download Register in Excel format).
Background
The procedure for authorising the placing on the market and use of feed additives is set out in Regulation 1831/2003.
Resources
EU Feed Additives register
Regulation 1831/2003 on additives for use in animal nutrition
EFSA (2013a) Scientific opinion on the safety and efficacy of CAROPHYLL® Red 10% (preparation of canthaxanthin) for all poultry for breeding purposes (chickens, turkeys and other poultry). EFSA Journal, 11(1): 3047.
EFSA (2013b) Scientific Opinion on the safety and efficacy of Enterococcus faecium (NCIMB 10415, DSM 22502, ATCC 53519 and ATCC 55593) as silage additives for all animal species. EFSA Journal, 11(10): 3363.
EFSA (2021a) Safety and efficacy of the feed additive consisting of Vitamin B2/Riboflavin produced by Eremothecium ashbyiCCTCCM 2019833 for all animal species (Hubei Guangji Pharmaceutical Co., Ltd. EFSA Journal, 19(3): 6462.
EFSA (2021b) Safety and efficacy of a feed additive consisting on the bacteriophages PCM F/00069, PCM F/00070, PCM F/00071 and PCM F/00097 infecting Salmonella Gallinarum B/00111 (Bafasal®) for all avian species (Proteon Pharmaceuticals S.A.). EFSA Journal, 19(5): e6534.
EFSA (2022a) Safety and efficacy of a feed additive consisting of sepiolite for all animal species (Sepiol S.A and Tolsa, S.A). EFSA Journal, 20(4): 7250.
EFSA (2022b) Assessment of the efficacy of two feed additives consisting of Enterococcus faecium ATCC 53519 and E. faecium ATCC 55593 for all animal species (FEFANA asbl). EFSA Journal, 20(10): e7603.
EFSA (2022c) Safety and efficacy of a feed additive consisting of 3-phytase produced by Komagataella phaffii (CECT 13171) (FSF10000/FLF1000) for poultry species, pigs for fattening and minor porcine species (FERTINAGRO BIOTECH S.L.). EFSA Journal, 20(11): e7614.
EFSA (2023a) Safety and efficacy of a feed additive consisting of the bacteriophages PCM F/00069, PCM F/00070, PCM F/00071 and PCM F/00097 (Bafasal®) for all avian species (Proteon Pharmaceuticals S.A.). EFSA Journal, 21(3): e7861
EFSA (2023b) Efficacy of the feed additives consisting of Enterococcus faecium ATCC 53519 and E. faecium ATCC 55593 for all animal species (FEFANA asbl). EFSA Journal, 21(7): e8166.
EFSA (2024a) Safety and efficacy of a feed additive consisting of an essential oil obtained from the fruit of Carum carvi L. (caraway oil) for all animal species (FEFANA asbl). EFSA Journal, 22(7): e8906.
EFSA (2024b) Safety and efficacy of a feed additive consisting of an essential oil obtained from the fruit of Apium graveolensL. (celery seed oil) for all animal species (FEFANA asbl). EFSA Journal, 22(7): e8907.
EFSA (2024c) Safety and efficacy of a feed additive consisting of a tincture derived from the leaves of Eucalyptus globulusLabill. (eucalyptus tincture) for all animal species (FEFANA asbl). EFSA Journal, 22(5): e8801.
EFSA (2024d) Safety of a feed additive consisting of sepiolite for all animal species (Sepiol S.A. and Tolsa S.A.). EFSA Journal, 22(6): e8850.
EFSA (2024e) Modification of the terms of authorisation regarding the additive consisting of liquid L-lysine base produced with Corynebacterium glutamicum NRRL B-67439 and NRRL B-67535 for all animal species (ADM specialty ingredients (Europe) B.V.). EFSA Journal, 22(7): e8950.
EFSA (2024f) Safety and efficacy of a feed additive consisting of an essential oil derived from the leaves of Salvia officinalis ssp. lavandulifolia (Vahl) Gams (Spanish sage oil) for use in all animal species (FEFANA asbl). EFSA Journal, 22(10): e9015.
EFSA (2024g) Safety and efficacy of a feed additive consisting of an essential oil derived from the flowering stems of Salvia sclarea L. (clary sage oil) for use in all animal species (FEFANA asbl). EFSA Journal, 22(11): e9016.
EFSA (2024h) Safety and efficacy of a feed additive consisting of Bacillus subtilis DSM 33862 and Lentilactobacillus buchneri DSM 12856 as a silage additive for all animal species (Lactosan GmbH & Co.KG). EFSA Journal, 22(11): e9070.
EFSA (2024i) Safety and efficacy of a feed additive consisting of an essential oil derived from the flowering tops of Lavandula angustifolia Mill. (lavender oil) for use in all animal species (FEFANA asbl). EFSA Journal, 22(11): e9017.
EFSA (2024j) Safety and efficacy of a feed additive consisting of an essential oil derived from the flowering tops of Thymbra capitata (L.) Cav. (Spanish type origanum oil) for use in all animal species (FEFANA asbl). EFSA Journal, 22(10): e9018.
EFSA (2024k) Safety of a feed additive consisting of 3-phytase produced with Komagataella phaffii CECT 13171 (FSF10000/FLF1000) for poultry species, pigs for fattening and minor porcine species for fattening (Fertinagro Biotech S.L.). EFSA Journal, 22(10): e9023.
EFSA (2024l) Safety and efficacy of a feed additive consisting of endo-1,4-β-xylanase produced with Trichoderma reesei CBS 114044 (ECONASE® XT) for pigs for fattening, laying hens and minor poultry species (AB Enzymes Finland Oy). EFSA Journal, 22(10): e9025.
EFSA (2024m) Safety and efficacy of a feed additive consisting of an essential oil derived from leaves and terminal branchlets of Melaleuca alternifolia (Maiden & Betche) Cheel (tea tree oil) for use in all animal species (FEFANA asbl). EFSA Journal, 22(10): e9026.
EFSA (2024n) Safety of a feed additive consisting of vitamin B2/riboflavin produced with Eremothecium ashbyi CCTCCM 2019833 for all animal species (Hubei Guangji Pharmaceutical Co., Ltd). EFSA Journal, 22(11): e9073.
EFSA (2024o) Assessment of the modification of the authorisation of the feed additive consisting of Saccharomyces cerevisiaeCNCM I-1077 for lambs and its extension of use to all ruminants and camelids reared for milk production/suckling/reproduction, all minor (young) ruminant species and camelids for fattening and Equidae other than horses (Lallemand SAS). EFSA Journal, 22(11): e9075.
EFSA (2024p) Safety and efficacy of a feed additive consisting of the bacteriophages PCM F/00069, PCM F/00070, PCM F/00071 and PCM F/00097 (Bafasal®) for all poultry (Proteon Pharmaceuticals S.A.). EFSA Journal, 22(12): e9132.
EFSA (2024q) Assessment of the feed additive consisting of Lactiplantibacillus plantarum NCIMB 40027 for all animal species for the renewal of its authorisation (Volac International ltd). EFSA Journal, 22(12): e9145.
EFSA (2024r) Safety and efficacy of a feed additive consisting of an essential oil derived from the leaves of Salvia officinalis L. (sage oil) for use in all animal species (FEFANA asbl). EFSA Journal, 22(12): e9135.
EFSA (2024s) Safety and efficacy of a feed additive consisting of Lactiplantibacillus plantarum NCIMB 41028 for all animal species (Genus Breeding Ltd.). EFSA Journal, 22(12): e9142.
EFSA (2024t) Safety and efficacy of a feed additive consisting of Lactiplantibacillus plantarum NCIMB 30148 for all animal species (Genus Breeding Ltd.). EFSA Journal, 22(12): e9143.
EFSA (2024u) Efficacy of the feed additives consisting of Enterococcus faecium ATCC 53519 and E. faecium ATCC 55593 as silage additives for all animal species (FEFANA asbl). EFSA Journal, 22(11): e9071.
EFSA (2025a) Safety and efficacy of a feed additive consisting of an essential oil derived from the aerial parts of Mentha × piperita L. (peppermint oil) for use in all animal species (FEFANA asbl). EFSA Journal, 23(1): e9076.
EFSA (2025b) Safety and efficacy of a feed additive consisting of Lactiplantibacillus plantarum NCIMB 30094 for all animal species (Volac International Ltd). EFSA Journal, 23(1): e9144.
EFSA (2025c) Modification of the terms of authorisation of the feed additive consisting of a preparation of canthaxanthin (CAROPHYLL® Red 10%) for breeder hens to include canthaxanthin produced with Yarrowia lipolytica CBS 146148 (DSM Nutritional Products Ltd). EFSA Journal, 23(1): e9133.
EFSA (2025d) Assessment of the feed additive consisting of biotin for all animal species for the renewal of its authorisation (ADISSEO, DSM Nutritional Products Ltd., NHU Europe GmbH). EFSA Journal, 23(2): e9250.
EFSA (2025e) Assessment of the feed additive consisting of endo-1,3(4)-beta-glucanase and endo-1,4-beta-xylanase (produced with Talaromyces versatilis IMI CC 378536) (Rovabio® Excel) for all poultry species, weaned piglets, pigs for fattening and sows for the renewal of its authorisation (Adisseo France SAS). EFSA Journal, 23(1): e9131.
Sources
Commission Implementing Regulations 2025/757, 2025/1254, 2025/1386, 2025/1390, 2025/1391, 2025/1392, 2025/1395, 2025/1400, 2025/1402, 2025/1403, 2025/1417, 2025/1418, 2025/1419, 2025/1423, 2025/1424, 2025/1426, 2025/1465, 2025/1468, 2025/1504, 2025/1523, 2025/1527
Notifications G/SPS/N/EU/857, G/SPS/N/EU/858, G/SPS/N/EU/863
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU authorises/reauthorises certain feed additives and withdraws others
Commission Implementing Regulations 2025/757, 2025/1254, 2025/1386, 2025/1390, 2025/1391, 2025/1392, 2025/1395, 2025/1400, 2025/1402, 2025/1403, 2025/1417, 2025/1418, 2025/1419, 2025/1423, 2025/1424, 2025/1426, 2025/1465, 2025/1468, 2025/1504, 2025/1523, 2025/1527
Notifications G/SPS/N/EU/857, G/SPS/N/EU/858, G/SPS/N/EU/863
What is changing and why?
Authorisations and reauthorisations
Between May and July 2025, the European Union (EU) authorised the feed additives listed in Table 1, and renewed authorisations of the feed additives listed in Table 2. These authorisations are based on opinions published by the European Food Safety Authority (EFSA). The conditions of use are described in the respective Regulations.
The EU has also informed the World Trade Organization Sanitary and Phytosanitary Measures (WTO SPS) Committee that it intends to renew the authorisations for eucalyptus tincture and celery seed essential oil as flavouring compounds in feed for certain food-producing animals, and to withdraw the authorisations for their use in feed for other animals.
Withdrawals
The EU also notified the WTO SPS Committee that it intends to withdraw a range of other feed additives. Feed additives are withdrawn from the market if no application for their renewal has been submitted before the deadline provided, or if an application was submitted but subsequently withdrawn. For those feed additives, a transitional period applies within which remaining stocks may be used up. In cases where applications have been submitted or withdrawn only for certain animal species or categories, the withdrawal only concerns those species and categories specified.
Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel (see Food and Feed Information Portal: Feed Additives > Download Register in Excel format).
Timeline
The authorisations remain valid until the end dates listed in Tables 1 and 2.
Transitional period for withdrawals:
- existing stocks, 12 months
- premixtures, 15 months
- compound feed, 24 months.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.