Feed additives: October–December 2025 authorisations and changes
- Feed additives
- Feed safety
Summary
Overview of the latest authorisations, reauthorisations, amendments, and withdrawals of feed additives and their use in animal nutrition in target animals.
Feed additives authorised, reauthorised, amended, and withdrawn October–December 2025
Commission Implementing Regulations 2025/2046, 2025/2171, 2025/2175, 2025/2176,2025/2183, 2025/2186, 2025/2491, 2025/2497, 2025/2498, 2025/2500, 2025/2502, 2025/2503, 2025/2505, 2025/2511, 2025/2566, 2025/2575, 2025/2576, 2025/2590
Update
Overview of the latest authorisations, reauthorisations, amendments, and withdrawals of feed additives and their use in animal nutrition in target animals.
Impacted Products
Prepared fodder
What is changing?
Authorisations
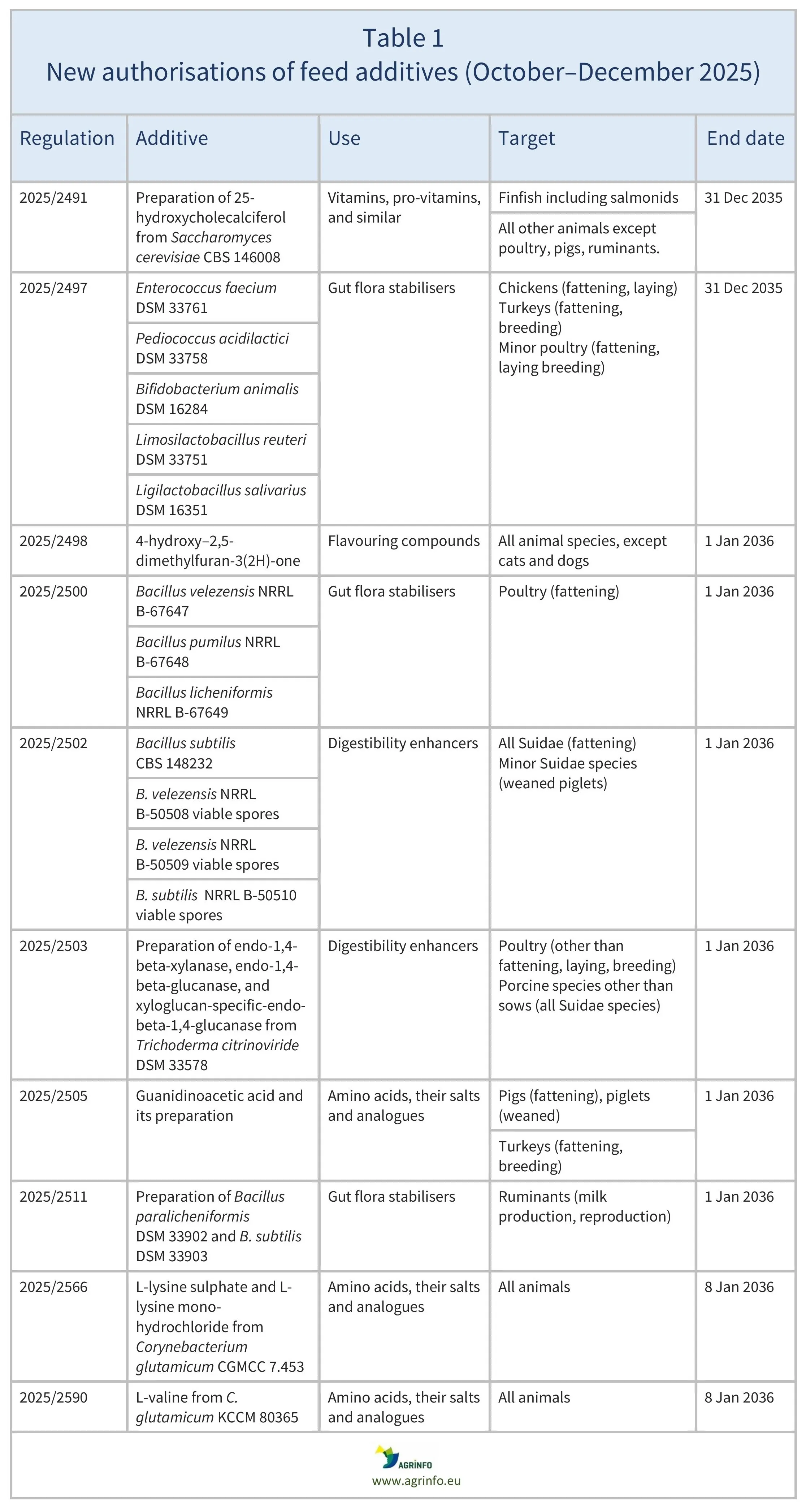
In October–December 2025, the European Union (EU) authorised the feed additives listed in Table 1, based on opinions published by the European Food Safety Authority (EFSA; see Resources). The conditions of use are described in the respective Regulations.
Reauthorisations
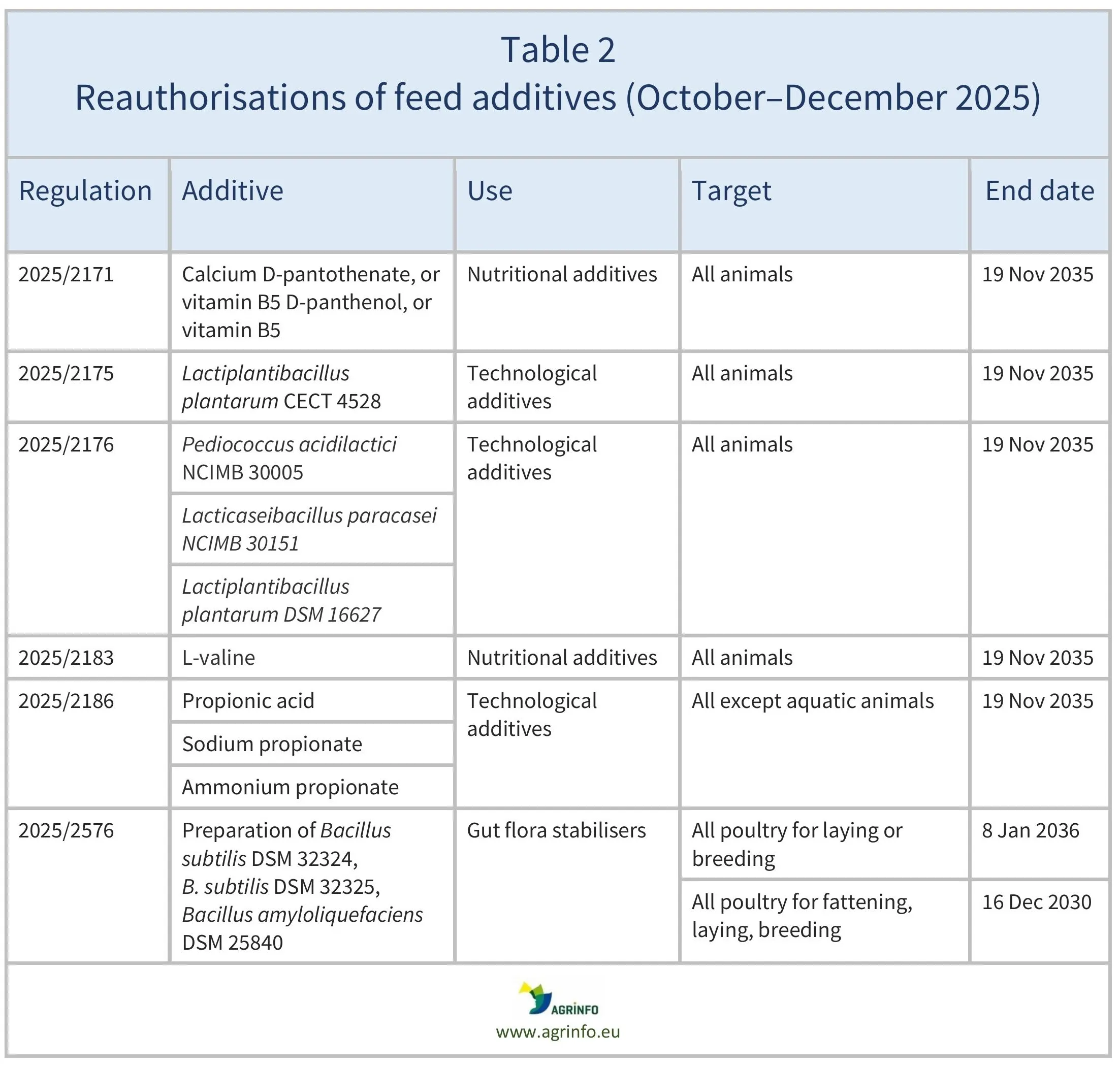
In October–December 2025, the EU renewed authorisations for the feed additives listed in Table 2, based on opinions published by EFSA (see Resources). The conditions of use are described in the respective Regulations.
Amendments
Regulation 2025/2046 authorises a new production route for canthaxanthin by fermentation with Yarrowia lipolytica CBS 146148. EFSA concluded that this production route is efficient as a colouring agent in feed for poultry for fattening and poultry reared for laying.
Regulation 2025/2171 reauthorises the use of calcium D-pantothenate (vitamin B5) and D-panthenol (provitamin B5), and adds the reference to the common name of the vitamin “vitamin B5”.
Regulation 2025/2576 authorises the use of a preparation of Bacillus subtilis DSM 32324, Bacillus subtilis DSM 32325, and Bacillus amyloliquefaciens DSM 25840 for all poultry species for laying or for breeding. It also introduces a new 10-fold increased concentration of the active agents in the additive for all poultry for fattening, laying, or breeding.
Withdrawals
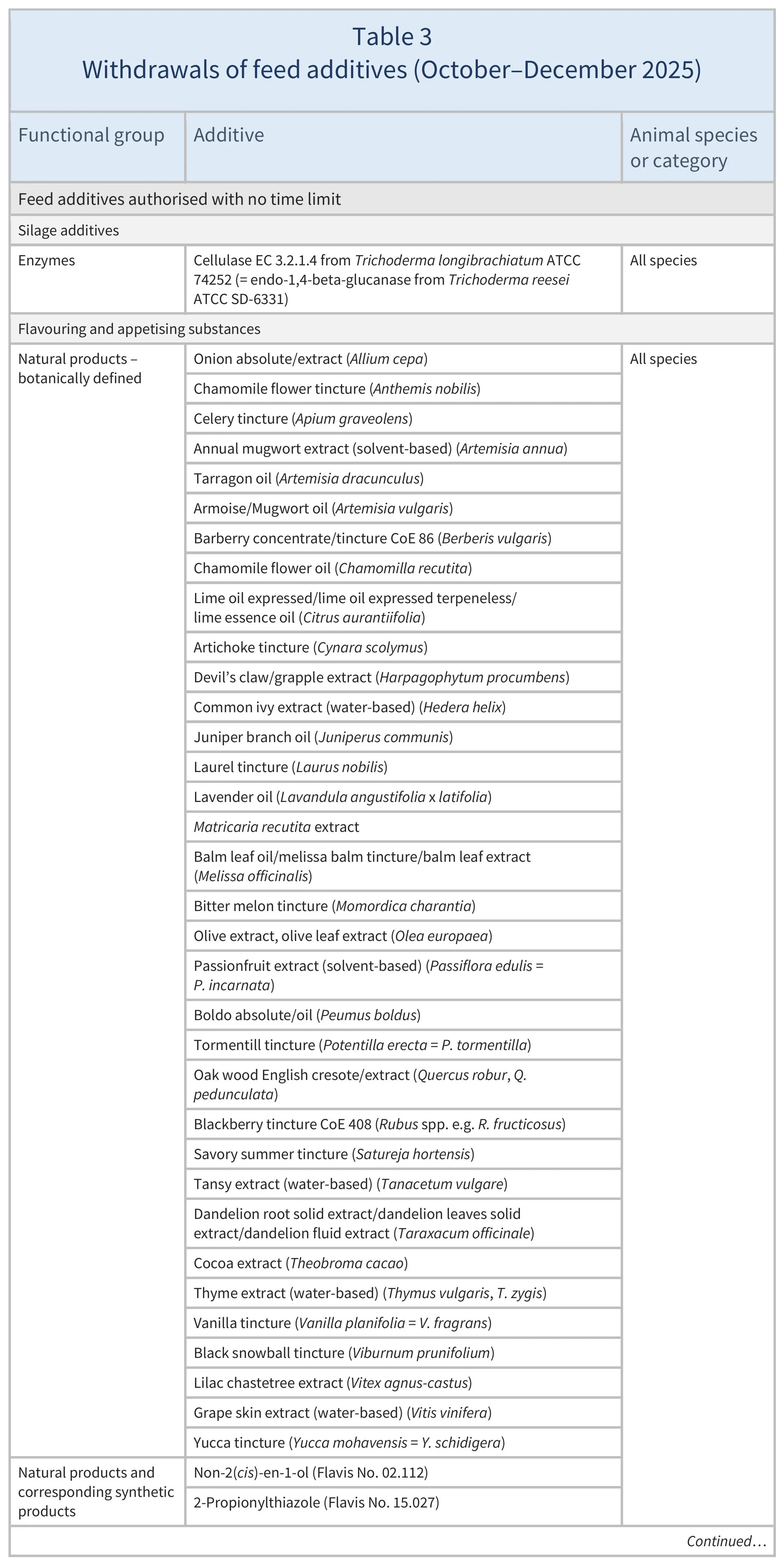
Regulation 2025/2575 withdraws the feed additives listed in Table 3. A transitional period applies for feed additives that were previously authorised without a time limit but now are withdrawn from the EU market.
Why?
Authorisations
Applications for the above authorisations were submitted and considered by the Reference Laboratory set up by the Feed Additives Regulation (1831/2003).
Withdrawals
Regulation (1831/2003) requires feed additives to be withdrawn from the market if no application has been submitted before the deadline provided, or if an application was submitted but subsequently withdrawn. In cases where applications have been submitted or withdrawn only for certain animal species or categories, the withdrawal only concerns those species and categories specified.
Timeline
The authorisations remain valid until the end dates listed in Tables 1 and 2.
Regulation 2025/2575 on withdrawals applies from 8 January 2026. Transitional period for withdrawals:
- existing stocks, 12 months
- premixtures, 15 months
- compound feed, 24 months.
What are the major implications for exporting countries?
Authorisations make more feed additives available on the EU market. Authorisations and renewals are valid for 10 years. All preparations and substances specified as feed additives must comply with the provisions of use specified in the Annex to each Regulation.
Recommended Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU can check the status of additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel (see Food and Feed Information Portal: Feed Additives > Download Register in Excel format).
Background
The procedure for authorising the placing on the market and use of feed additives is set out in Regulation 1831/2003.
Resources
EU Feed Additives register
Regulation 1831/2003 on additives for use in animal nutrition
- EFSA (2020) Safety and efficacy of GalliPro® Fit (Bacillus subtilis DSM 32324, Bacillus subtilis DSM 32325 and Bacillus amyloliquefaciens DSM 25840) for all poultry species for fattening or reared for laying/breeding. EFSA Journal, 18(4): 6094.
- EFSA (2022) Safety and efficacy of a feed additive consisting of guanidinoacetic acid for all animal species (Alzchem Trostberg GmbH). EFSA Journal, 20(5): 7269.
- EFSA (2022) Safety and efficacy of a feed additive consisting of endo-1,4-beta xylanase, endo-1,4-beta-glucanase and xyloglucan-specific-endo-beta-1,4-glucanase produced by Trichoderma citrinoviride DSM 33578 (Huvezym® neXo 100 G/L) for all poultry species, ornamental birds and piglets (weaned and suckling) (Huvepharma EOOD). EFSA Journal, 20(12): 7702.
- EFSA (2023) Safety and efficacy of a feed additive consisting of 25-hydroxycholecalciferol produced with Saccharomyces cerevisiae CBS 146008 for pigs and poultry for the renewal of its authorisation (DSM Nutritional Products Sp. z.o.o). EFSA Journal, 21(8): 8168.
- EFSA (2023) Safety and efficacy of a feed additive consisting of Enterococcus faecium DSM 33761, Pediococcus acidilactici DSM 33758, Bifidobacterium animalis DSM 16284, Limosilactobacillus reuteri DSM 33751 and Ligilactobacillus salivarius DSM 16351 (Biomin® C5) for chickens for fattening, chickens reared for laying, turkeys for fattening, turkeys reared for breeding and minor poultry species for fattening and reared for laying/breeding (Biomin GmbH). EFSA Journal, 21(10): 8354.
- EFSA (2023) Modification of the terms of authorisation of the feed additive consisting of canthaxanthin for chickens for fattening, minor poultry species for fattening, laying poultry and poultry reared for laying, ornamental fish and ornamental birds and ornamental (DSM Nutritional Products Ltd). EFSA Journal, 23(1): e9134.
- EFSA (2024) Safety and efficacy of a feed additive consisting of endo-1,4-β xylanase, endo-1,4-β-glucanase and xyloglucan-specific-endo-β-1,4-glucanase produced by Trichoderma citrinoviride DSM 33578 (Huvezym® neXo) for all Suidae (Huvepharma EOOD). EFSA Journal 22(3): e8643.
- EFSA (2024) Assessment of the feed additive consisting of calcium D-pantothenate for all animal species for the renewal of its authorisation (BASF SE). EFSA Journal, 22(7): e8901.
- EFSA (2024) Assessment of the feed additive consisting of sodium propionate for all terrestrial animal species for the renewal of its authorisation (BASF SE). EFSA Journal, 22(8): e8938.
- EFSA (2024) Assessment of the feed additive consisting of propionic acid for all terrestrial animal species for the renewal of its authorisation (Eastman Chemical B.V., Perstorp AB, Dow Europe GmbH, BASF SE). EFSA Journal, 22(10): e9020.
- EFSA (2024) Assessment of the feed additive consisting of ammonium propionate for all terrestrial animal species for the renewal of its authorisation (BASF SE, Taminco Finland Oy). EFSA Journal, 22(10): e9068.
- EFSA (2024) Assessment of the feed additive consisting of Lacticaseibacillus paracasei NCIMB 30151 for all animal species for the renewal of its authorisation (Microferm Ltd). EFSA Journal, 22(11): e9074.
- EFSA (2025) Assessment of the feed additive consisting of Pediococcus acidilactici NCIMB 30005 for all animal species for the renewal of its authorisation (Microferm Ltd). EFSA Journal, 23(1): e9146.
- EFSA (2025) Assessment of the feed additive consisting of Lactiplantibacillus plantarum DSM 16627 for all animal species for the renewal of its authorisation (Microferm Ltd). EFSA Journal, 23(2): e9248.
- EFSA (2025) Assessment of the feed additive consisting of L-valine produced by fermentation with Corynebacterium glutamicum KCCM 80058 for all animal species for the renewal of its authorisation (CJ Europe GmbH). EFSA Journal, 23(2): e9251.
- EFSA (2025) Assessment of the feed additives calcium D-pantothenate and D-panthenol for all animal species for the renewal of their authorisation (DSM Nutritional products Ltd). EFSA Journal, 23(2): e9252.
- EFSA (2025) Safety and efficacy of a feed additive consisting of protease and Bacillus velezensis NRRL B-50508, B. velezensis NRRL B-50509 and B. subtilis NRRL B-50510 (Syncra® SWI 201 TPT) for pigs for fattening and other growing porcine species (Danisco (UK) Ltd). EFSA Journal, 23(2): e9265.
- EFSA (2025) Safety and efficacy of a feed additive consisting of L-lysine sulfate produced by fermentation with Corynebacterium glutamicum CGMCC 7.453 for all animal species (Eppen Europe SAS). EFSA Journal, 23(4): e9343.
- EFSA (2025) Safety and efficacy of a feed additive consisting of L-lysine monohydrochloride produced with Corynebacterium glutamicum CGMCC 7.453 for all animal species (Eppen Europa SAS). EFSA Journal, 23(4): e9345.
- EFSA (2025) Safety and efficacy of a feed additive consisting of L-valine produced with Corynebacterium glutamicum KCCM 80365 for all animal species (CJ Europe GmbH). EFSA Journal, 23(4): e9348.
- EFSA (2025) Safety and efficacy of a feed additive consisting of guanidinoacetic acid (Creamino®) for turkeys for fattening and reared for breeding (Alzchem Trostberg GmbH). EFSA Journal, 23(4): e9349.
- EFSA (2025) Efficacy of a feed additive consisting of guanidinoacetic acid (Creamino®) for weaned piglets and pigs for fattening in water for drinking (Alzchem Trostberg GmbH). EFSA Journal, 23(4): e9350.
- EFSA (2025) Safety and efficacy of a feed additive consisting of Bacillus subtilis DSM 32324, Bacillus subtilis DSM 32325 and Bacillus amyloliquefaciens DSM 25840 (GalliPro® Fit & GalliPro® Fit 10) for all poultry (Chr. Hansen A/S). EFSA Journal, 23(4): e9361.
- EFSA (2025) Safety and efficacy of a feed additive consisting of Bacillus paralicheniformis DSM 33902 and Bacillus subtilis DSM 33903 (Bovacillus™) for dairy cows and other dairy ruminants (Chr. Hansen A/S). EFSA Journal, 23(4): e9426.
- EFSA (2025) Assessment of the feed additive consisting of Lactiplantibacillus plantarum CECT 4528 for all animal species for the renewal of its authorisation (Centro Sperimentale del Latte S.r.l.). EFSA Journal, 23(2): e9427.
- EFSA (2025) Efficacy of a feed additive consisting of Enterococcus faecium DSM 33761, Pediococcus acidilactici DSM 33758, Bifidobacterium animalis DSM 16284, Limosilactobacillus reuteri DSM 33751, Ligilactobacillus salivarius DSM 16351 (Biomin® C5) as a zootechnical additive for poultry for fattening and reared for laying/breeding (Biomin GmbH). EFSA Journal, 23(5): e9459.
- EFSA (2025) Efficacy of a feed additive consisting of endo-1,4-beta-xylanase, endo-1,4-beta-glucanase and xyloglucan-specific-endo-beta-1,4-glucanase produced by Trichoderma citrinoviride DSM 33578 (Huvezym® neXo) for all porcine species and all poultry (Huvepharma EOOD). EFSA Journal, 23(6): e9460.
- EFSA (2025) Safety and efficacy of a feed additive consisting of Bacillus velezensis NRRL B-67647, Bacillus pumilus NRRL B-67648 and Bacillus licheniformis NRRL B-67649 (Microsaf®) for chickens for fattening, other poultry for fattening and ornamental birds (S.I. Lesaffre). EFSA Journal, 23(6): e9465.
- EFSA (2025) Safety and efficacy of a feed additive consisting of 25-hydroxycholecalciferol produced with Saccharomyces cerevisiae CBS 146008 for salmonids, other fish species and all other animal species (except poultry, pigs and all ruminants) (DSM Nutritional Products Ltd). EFSA Journal, 23(6): e9479.
- EFSA (2025) Safety and efficacy of a feed additive consisting of 4-hydroxy-2,5-dimethylfuran-3(2H)-one for all animal species other than dogs and cats (ADISSEO France SAS, ADM International Sàrl, Laboratoires Phodé S.A.S., LUCTA S.A., Norel, S.A.). EFSA Journal, 23(7): e9538.
Sources
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
Feed additives authorised, reauthorised, amended, and withdrawn October–December 2025
Commission Implementing Regulations 2025/2046, 2025/2171, 2025/2175, 2025/2176, 2025/2183, 2025/2186, 2025/2491, 2025/2497, 2025/2498, 2025/2500, 2025/2502, 2025/2503, 2025/2505, 2025/2511, 2025/2566, 2025/2575, 2025/2576, 2025/2590
What is changing and why?
In October–December 2025, the European Union (EU) authorised the feed additives listed in Table 1, based on opinions published by the European Food Safety Authority. The conditions of use are described in the respective Regulations.
Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU can check the status of additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel (see Food and Feed Information Portal: Feed Additives > Download Register in Excel format).
Timeline
The authorisations remain valid until the end dates listed in Tables 1 and 2.
Regulation 2025/2575 on withdrawals applies from 8 January 2026. Transitional period for withdrawals:
- existing stocks, 12 months
- premixtures, 15 months
- compound feed, 24 months.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.