Feed additives: Reauthorisations, amendments, and corrections
- Feed additives
Summary
This overview describes the EU's latest reauthorisations of feed additives and their use in animal nutrition in target animals, as well as some amendments and corrections to existing authorisations.
EU reauthorises certain feed additives, also makes some amendments and corrections
Commission Implementing Regulations 2024/1810, 2024/1839, 2024/1989, 2024/2039, 2024/2040
Update
This overview describes the EU's latest reauthorisations of feed additives and their use in animal nutrition in target animals, as well as some amendments and corrections to existing authorisations.
Impacted Products
Feed additives, prepared fodder
What is changing?
Reauthorisations
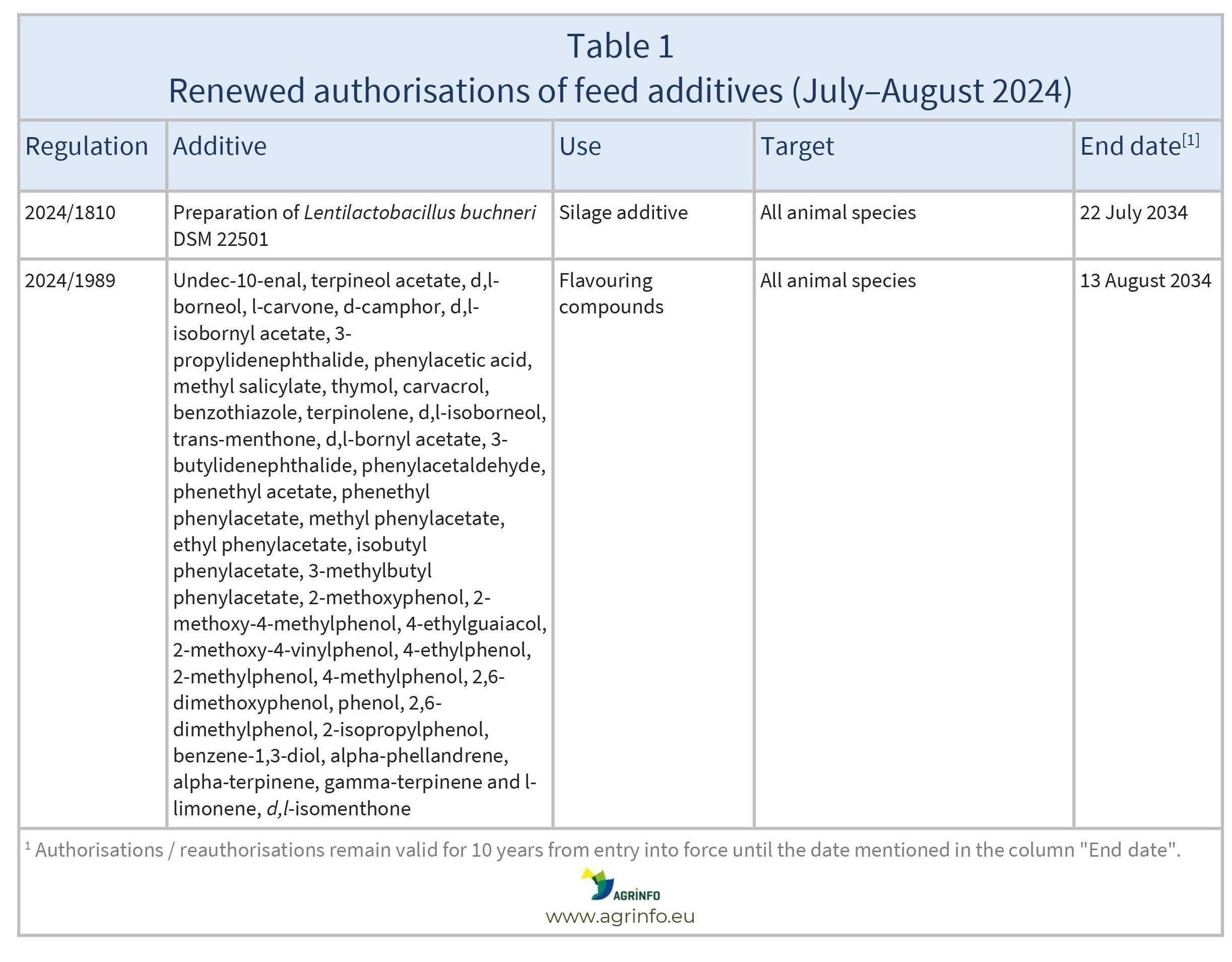
In July and August 2024, the EU reauthorised the feed additives listed in Table 1.
These authorisations are based on opinions published by the European Food Safety Authority (EFSA) (2023, 2024). The conditions of use are described in the respective Regulations.
Amendments and corrections
- Regulation 2024/1839 corrects 2023/2846 by deleting Schinopsis lorentzii as a source of red quebracho extract.
- Regulation 2024/2039 deletes the feed additives sunflower absolute, sunflower oil, and sunflower tincture from Helianthus annuus from the Annex to Regulation 2023/1173.
- Regulation 2024/2040 establishes transitional measures for the renewal of the authorisation of a preparation of 25-hydroxycholecalciferol produced by Saccharomyces cerevisiae CBS 146008.
Why?
Reauthorisations
Applications for the above authorisations were submitted and considered by the Reference Laboratory set up by the Feed Additives Regulation (1831/2003).
Amendments and corrections
- Regulation (EU) 2023/2846 authorised red quebracho extract as feed additive for all animal species, but erroneously authorised Schinopsis lorentzii as a source. This was not requested in the application for authorisation. Schinopsis lorentzii is therefore deleted to prevent the marketing of red quebracho extract from that source in the EU.
- Sunflower absolute, sunflower oil, and sunflower tincture from Helianthus annuus were withdrawn from the EU market as feed additives for all animal species. Those feed additives were erroneously included in the Annex to Regulation 2023/1173.
- Regulation 2024/1070 set stricter terms of authorisation for the feed additive 25-hydroxycholecalciferol produced with Saccharomyces cerevisiae CBS 146008, but failed to lay down transitional measures to allow interested parties to adapt to these stricter terms of authorisation.
Timeline
The reauthorisations remain valid until the end dates listed in Table 1.
- Regulation 2024/1839 applies since 5 July. The feed additive, and premixtures, compound feed, and feed materials containing it, produced and labelled before 5 August 2024, may continue to be placed on the market and used until 5 January 2025.
- Regulation 2024/2039 applies since 31 July 2024.
- Regulation 2024/2040 applies retrospectively since 5 May 2024. The feed additive and premixtures containing that additive, if intended for chickens for fattening, turkeys for fattening, other poultry and pigs, and produced and labelled before 5 November 2024, may be used until stocks are exhausted.
What are the major implications for exporting countries?
With these new authorisations, more feed additives will be available on the market. Authorisations and renewals are valid for 10 years. The use of all preparations and substances specified as feed additives must comply with the provisions of use specified in the Annex to each Regulation.
Recommended Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of the feed additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel format (see foot of Food and Feed Information Portal webpage).
Background
The procedure for authorising the placing on the market and use of feed additives is set out in Regulation (EC) 1831/2003. For the latest updates on feed additives see the EU Feed Additives register.
Resources
Regulation 1831/2003 on additives for use in animal nutrition
EFSA (2024) Assessment of the feed additive consisting of Lentilactobacillus buchneri (formerly Lactobacillus buchneri) DSM 22501 for all animal species for the renewal of its authorisation (Chr. Hansen A/S). EFSA Journal, 22(1): e8541.
EFSA (2023) Safety of 41 flavouring compounds providing a herbal flavour and belonging to different chemical groups for use as feed additives in all animal species (FEFANA asbl). EFSA Journal, 21(10): 8340.
Sources
Commission Implementing Regulations:
- 2024/1810 concerning the renewal of the authorisation of a preparation of Lentilactobacillus buchneri DSM 22501 as a feed additive for all animal species and amending Implementing Regulation (EU) No 1113/2013
- 2024/1839 correcting Implementing Regulation (EU) 2023/2846 by deleting Schinopsis lorentzii (Griseb.) Engl. as a source of the feed additive red quebracho extract
- 2024/1989 concerning the authorisation of undec-10-enal, terpineol acetate, d,l-borneol, l-carvone, d-camphor, d,l-isobornyl acetate, 3-propylidenephthalide, phenylacetic acid, methyl salicylate, thymol, carvacrol, benzothiazole, terpinolene, d,l-isoborneol, trans-menthone, d,l-bornyl acetate, 3-butylidenephthalide, phenylacetaldehyde, phenethyl acetate, phenethyl phenylacetate, methyl phenylacetate, ethyl phenylacetate, isobutyl phenylacetate, 3-methylbutyl phenylacetate, 2-methoxyphenol, 2-methoxy-4-methylphenol, 4-ethylguaiacol, 2-methoxy-4-vinylphenol, 4-ethylphenol, 2-methylphenol, 4-methylphenol, 2,6-dimethoxyphenol, phenol, 2,6-dimethylphenol, 2-isopropylphenol, benzene-1,3-diol, alpha-phellandrene, alpha-terpinene, gamma-terpinene and l-limonene as feed additives for all animal species and amending Implementing Regulation (EU) 2018/245 as regards the terms of authorisation of d,l-isomenthone as a feed additive for all animal species
- 2024/2039 correcting Implementing Regulation (EU) 2023/1173 by deleting feed additives from the Annex thereto
- 2024/2040 amending Implementing Regulation (EU) 2024/1070 as regards the establishment of transitional measures for the renewal of the authorisation of a preparation of 25-hydroxycholecalciferol produced by Saccharomyces cerevisiae CBS 146008
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU reauthorises certain feed additives, also makes some amendments and corrections
Commission Implementing Regulations 2024/1810, 2024/1839, 2024/1989, 2024/2039, 2024/2040
What is changing and why?
Reauthorisations
In July and August 2024, the EU authorised or reauthorised the feed additives listed in Table 1. These authorisations are based on opinions published by the European Food Safety Authority (EFSA). The conditions of use are described in the respective Regulations.
Amendments and corrections
- Regulation 2024/1839 deletes Schinopsis lorentzii as a source of red quebracho extract because it was erroneously authorised as a source, despite not being requested as such in the application for authorisation.
- Regulation 2024/2039 deletes the feed additives sunflower absolute, sunflower oil, and sunflower tincture from Helianthus annuus from the Annex to Regulation 2023/1173 because they were erroneously included in that Annex despite being withdrawn from the EU market as feed additives for all animal species.
- Regulation 2024/2040 establishes transitional measures for the renewal of the authorisation of a preparation of 25-hydroxycholecalciferol produced by Saccharomyces cerevisiae CBS 146008 because an earlier regulation (2024/1070) failed to lay down transitional measures to allow interested parties to adapt to these stricter terms of authorisation.
Timeline
The reauthorisations remain valid until the end dates listed in Table 1.
- Regulation 2024/1839 applies since 5 July. The feed additive, and premixtures, compound feed, and feed materials containing it, produced and labelled before 5 August 2024, may continue to be placed on the market and used until 5 January 2025.
- Regulation 2024/2039 applies since 31 July 2024.
- Regulation 2024/2040 applies retrospectively since 5 May 2024. The feed additive and premixtures containing that additive, if intended for chickens for fattening, turkeys for fattening, other poultry and pigs, and produced and labelled before 5 November 2024, may be used until stocks are exhausted.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.