Feed additives: September 2024 authorisations and reauthorisations
- Feed additives
Summary
An overview of the latest authorisations and reauthorisations of feed additives, and their use in animal nutrition in target animals.
EU authorises and reauthorises certain feed additives
Commission Implementing Regulations 2024/2177, 2024/2180, 2024/2184, 2024/2185, 2024/2385, 2024/2388, 2024/2389, 2024/2393, 2024/2412, 2024/2414, 2024/2427, 2024/2441, 2024/2464
Update
An overview of the latest authorisations and reauthorisations of feed additives, and their use in animal nutrition in target animals.
Impacted Products
Feed additives, prepared fodder
What is changing?
Authorisations
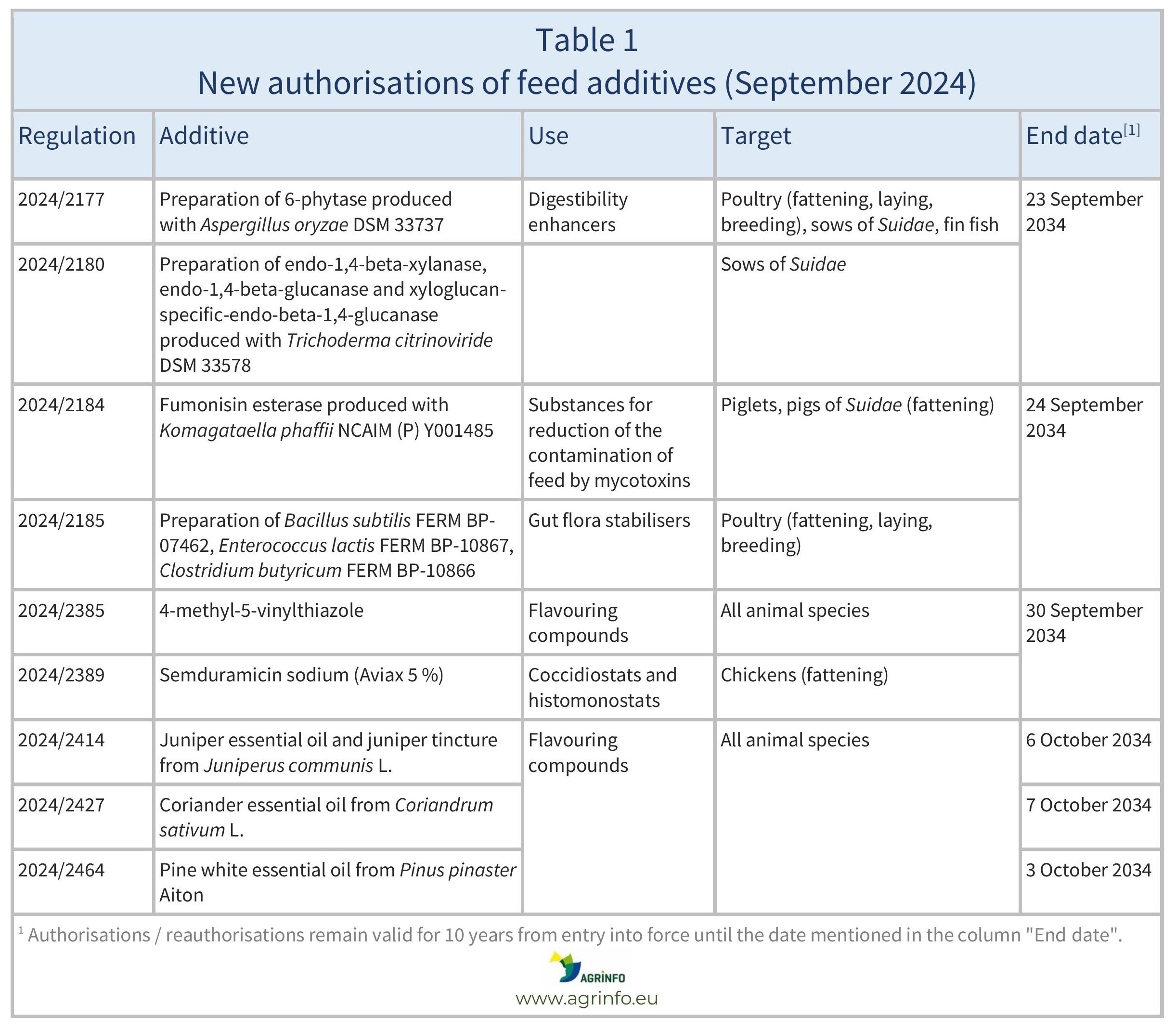
In September 2024, the EU authorised the feed additives listed in Table 1.
These authorisations are based on opinions published by the European Food Safety Authority (EFSA). The conditions of use are described in the respective Regulations.
Reauthorisations
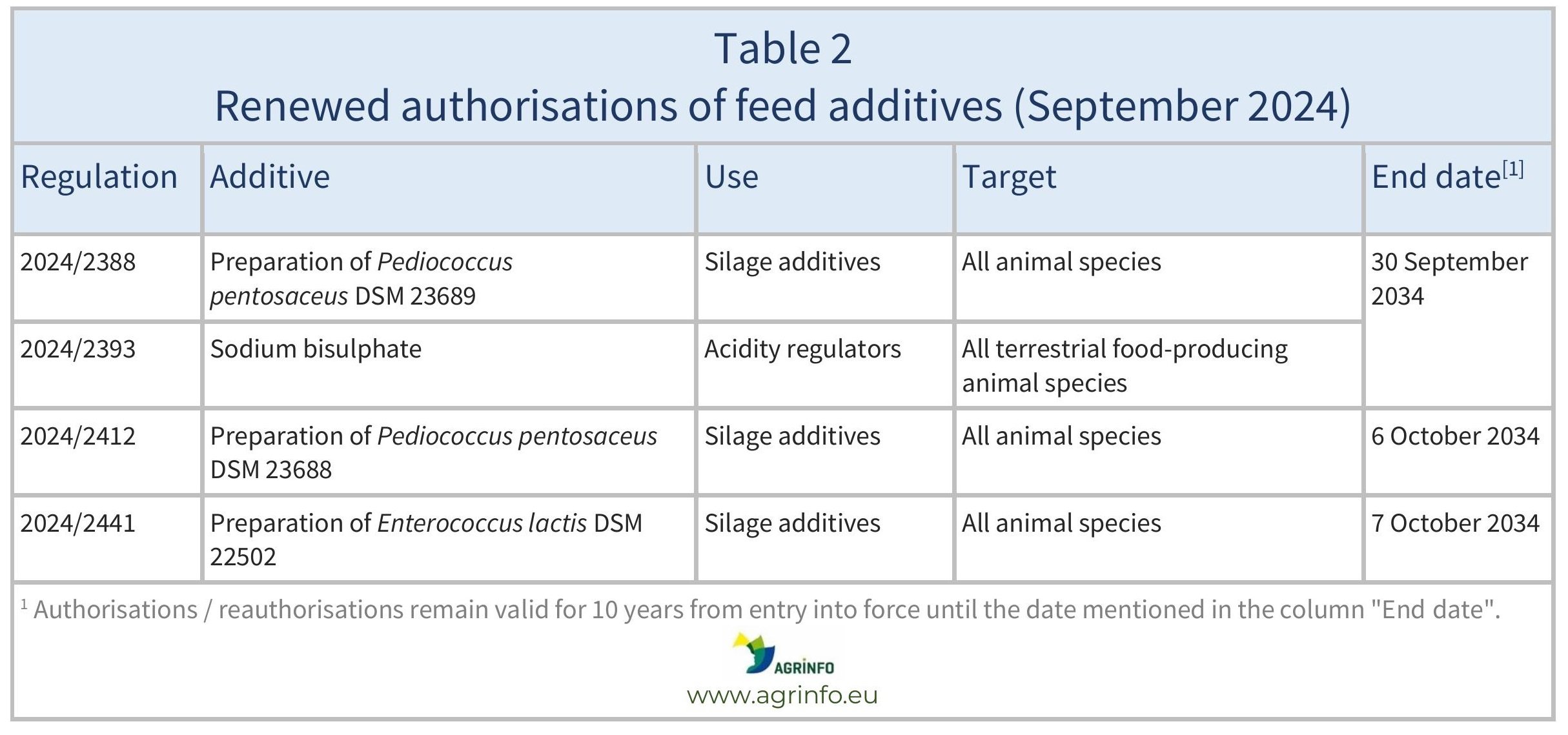
In September 2024, the EU reauthorised the feed additives listed in Table 2.
These authorisations are based on opinions published by the European Food Safety Authority (EFSA). The conditions of use are described in the respective Regulations.
Why?
Applications for the above authorisations and reauthorisations were submitted and considered by the Reference Laboratory set up by the Feed Additives Regulation (1831/2003).
Timeline
The authorisations and reauthorisations remain valid until the end dates listed in Tables 1 and 2.
What are the major implications for exporting countries?
With these authorisations, more feed additives will be available on the market. Authorisations and renewals are valid for 10 years. The use of all preparations and substances specified as feed additives must comply with the provisions of use specified in the Annex to each Regulation.
Recommended Actions
Non-EU countries producing feed additives, compound feed, and feed materials for export to the EU are recommended to check the status of the feed additives in the EU Feed Additives register.
To be able to filter and to see more information, it is advised to download the register in Excel format (see foot of Food and Feed Information Portal webpage).
Background
The procedure for authorising the placing on the market and use of feed additives is set out in Regulation (EC) 1831/2003. For the latest updates on feed additives see the EU Feed Additives register.
Resources
EU Feed Additives register
Regulation 1831/2003 on additives for use in animal nutrition
- EFSA (2022) Safety and efficacy of a feed additive consisting of Bacillus subtilis FERM BP-07462, Enterococcus lactis FERM BP-10867 and Clostridium butyricum FERM BP-10866 (BIO-THREE®) for chickens for fattening, chickens reared for laying, turkeys for fattening, turkeys reared for breeding, all avian species for rearing/fattening to slaughter and all avian species reared for laying or breeding to point of lay (TOA BIOPHARMA Co., Ltd.). EFSA Journal, 20(6): 7342.
- EFSA (2022) Safety of a feed additive consisting of semduramicin sodium (Aviax 5%) for chickens for fattening (Phibro Animal Health s.a.). EFSA Journal, 20(8): 7432.
- EFSA (2023) Safety and efficacy of a feed additive consisting of an essential oil obtained from the oleoresin of Pinus pinaster Aiton (pine white oil) for use in all animal species (FEFANA asbl). EFSA Journal, 21(2): 7952.
- EFSA (2022) Safety and efficacy of feed additives consisting of an essential oil and tincture from the berries of Juniperus communis L. (juniper oil and juniper tincture) for use in all animal species (FEFANA asbl). EFSA Journal, 21(4): 7977.
- EFSA (2022) Safety and efficacy of the feed additive4-methyl-5-vinylthiazole [15.018] belonging to chemical group 29 for all animal species (FEFANA asbl). EFSA Journal, 21(6): 8051.
- EFSA (2022) Safety and efficacy of a feed additive consisting of an essential oil obtained from the fruit of Coriandrum sativum L. (coriander oil) (FEFANA asbl). EFSA Journal, 21(10): 8349.
- EFSA (2023) Efficacy of a feed additive consisting of Bacillus subtilis FERM BP‐07462, Enterococcus lactis FERM BP‐10867 and Clostridium butyricum FERM BP‐10866 (BIO‐THREE®) for chickens for fattening and reared for laying, turkeys for fattening and reared for breeding, and all avian species for rearing/fattening or reared for laying/breeding (TOA BIOPHARMA Co., Ltd.). EFSA Journal, 21(11): 8343.
- EFSA (2024) Assessment of the feed additive consisting of Pediococcus pentosaceus DSM 23688 for all animal species for the renewal of its authorisation (Chr. Hansen A/S). EFSA Journal, 22(2): e8619.
- EFSA (2024) Assessment of the feed additive consisting of Pediococcus pentosaceus DSM 23689 for all animal species for the renewal of its authorisation (Chr. Hansen A/S). EFSA Journal, 22(2): e8620.
- EFSA (2024) Safety and efficacy of a feed additive consisting of fumonisin esterase produced with Komagataella phaffii NCAIM (P) Y001485 for all pigs (piglets, pigs for fattening, sows and minor growing and reproductive porcine species) (Dr. Bata Ltd.). EFSA Journal, 22(3): e8614.
- EFSA (2024) Assessment of the feed additive consisting of Enterococcus lactis DSM 22502 for all animal species for the renewal of its authorisation (Chr. Hansen A/S). EFSA Journal, 22(3): e8621.
- EFSA (2024) Safety and efficacy of a feed additive consisting of endo-1,4-β xylanase, endo-1,4-β-glucanase and xyloglucan-specific-endo-β-1,4-glucanase produced by Trichoderma citrinoviride DSM 33578 (Huvezym® neXo) for all Suidae (Huvepharma EOOD). EFSA Journal, 22(3): e8643.
- EFSA (2024) Safety and efficacy of a feed additive consisting of sodium bisulphate for all animal species except aquatic animals (Grillo Werke AG & Jones-Hamilton Co.). EFSA Journal, 22(3): e8644.
- EFSA (2024) Safety and efficacy of a feed additive consisting of 6‐phytase produced by Aspergillus oryzae DSM 33737 (HiPhorius™) for all poultry, all Suidae and all fin fish (DSM Nutritional Products Ltd). EFSA Journal, 22(3): e8663.
Sources
Commission Implementing Regulations:
- 2024/2464 concerning the authorisation of pine white essential oil from Pinus pinaster Aiton as a feed additive for all animal species
- 2024/2441 concerning the renewal of the authorisation of a preparation of Enterococcus lactis DSM 22502 as a feed additive for all animal species and repealing Implementing Regulation (EU) No 304/2014
- 2024/2427 concerning the authorisation of coriander essential oil from Coriandrum sativum L. as a feed additive for all animal species
- 2024/2414 concerning the authorisation of juniper essential oil and juniper tincture from Juniperus communis L. as feed additives for all animal species
- 2024/2412 concerning the renewal of the authorisation of a preparation of Pediococcus pentosaceus DSM 23688 as a feed additive for all animal species and amending Implementing Regulation (EU) No 84/2014
- 2024/2393 concerning the renewal of the authorisation of sodium bisulphate and the authorisation of new uses of that substance as a feed additive for certain animal species
- 2024/2389 concerning the authorisation of a preparation of semduramicin sodium (Aviax 5 %) as a feed additive for chickens for fattening (holder of authorisation: Phibro Animal Health s.a.) and repealing Regulation (EC) No 1443/2006
- 2024/2388 concerning the renewal of the authorisation of a preparation of Pediococcus pentosaceus DSM 23689 as a feed additive for all animal species and amending Implementing Regulation (EU) No 84/2014
- 2024/2385 concerning the authorisation of 4-methyl-5-vinylthiazole as a feed additive for all animal species
- 2024/2185 of 3 September 2024 concerning the authorisation of a preparation of Bacillus subtilis FERM BP-07462, Enterococcus lactis FERM BP-10867 and Clostridium butyricum FERM BP-10866 as a feed additive for all poultry species for fattening, all poultry species reared for laying or breeding and ornamental birds (holder of authorisation: Toa Biopharma Co., Ltd.)
- 2024/2184 concerning the authorisation of a preparation of fumonisin esterase produced with Komagataella phaffii NCAIM (P) Y001485 as a feed additive for piglets and pigs for fattening of all Suidae species
- 2024/2180 concerning the authorisation of a preparation of endo-1,4-beta-xylanase, endo-1,4-beta-glucanase and xyloglucan-specific-endo-beta-1,4-glucanase produced with Trichoderma citrinoviride DSM 33578 as a feed additive for sows of all Suidae species (holder of authorisation: Huvepharma EOOD)
- 2024/2177 concerning the authorisation of a preparation of 6-phytase produced with Aspergillus oryzae DSM 33737 as a feed additive for all poultry species for fattening or reared for laying or reared for breeding, sows of all Suidae species and all fin fish (holder of authorisation: DSM Nutritional Products Ltd, represented by DSM Nutritional Products Sp. z o.o.)
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU authorises and reauthorises certain feed additives
Commission Implementing Regulations 2024/2177, 2024/2180, 2024/2184, 2024/2185, 2024/2385, 2024/2388, 2024/2389, 2024/2393, 2024/2412, 2024/2414, 2024/2427, 2024/2441, 2024/2464
What is changing and why?
Authorisations and reauthorisations
In September 2024, the EU authorised or reauthorised feed additives listed in Tables 1 and 2. These authorisations are based on opinions published by the European Food Safety Authority (EFSA). The conditions of use are described in the respective Regulations.
Timeline
The authorisations and reauthorisations remain valid until the end dates listed in Tables 1 and 2.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.