Flavourings: Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one
- Food additives
Summary
The European Union (EU) has added Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one to the list of flavourings that can be used in foods.
EU authorises flavourings Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1
Commission Regulation (EU) 2025/1112 of 4 June 2025 amending Annex I to Regulation (EC) No 1334/2008 as regards the inclusion of Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one in the Union list of flavourings
Update
The European Union (EU) has added Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one to the list of flavourings that can be used in foods.
Impacted Products
Milk products, cheese and cheese products, edible ices, cocoa and chocolate products, confectionery, chewing gum, breakfast cereals, meat preparations, meat products, table-top sweeteners, herbs, spices, seasonings, soups and broths, protein products, non-alcoholic beverages, potato-, cereal-, flour-, or starch-based snacks, desserts, flavoured drinks
What is changing?
The EU has added Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one to the list of flavourings that can be used in foods.
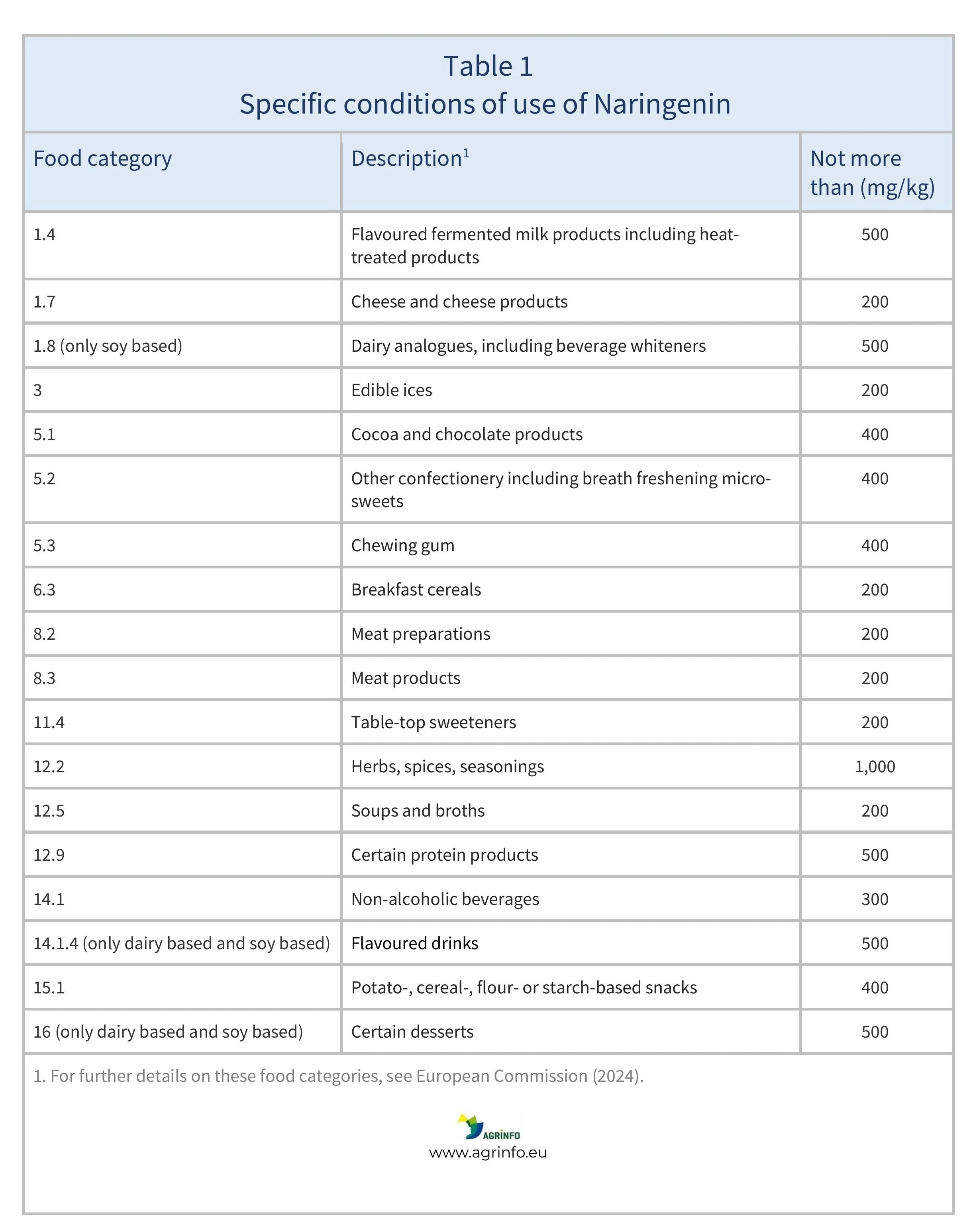
- Naringenin is only authorised to be used in certain categories of food, as detailed in Table 1.
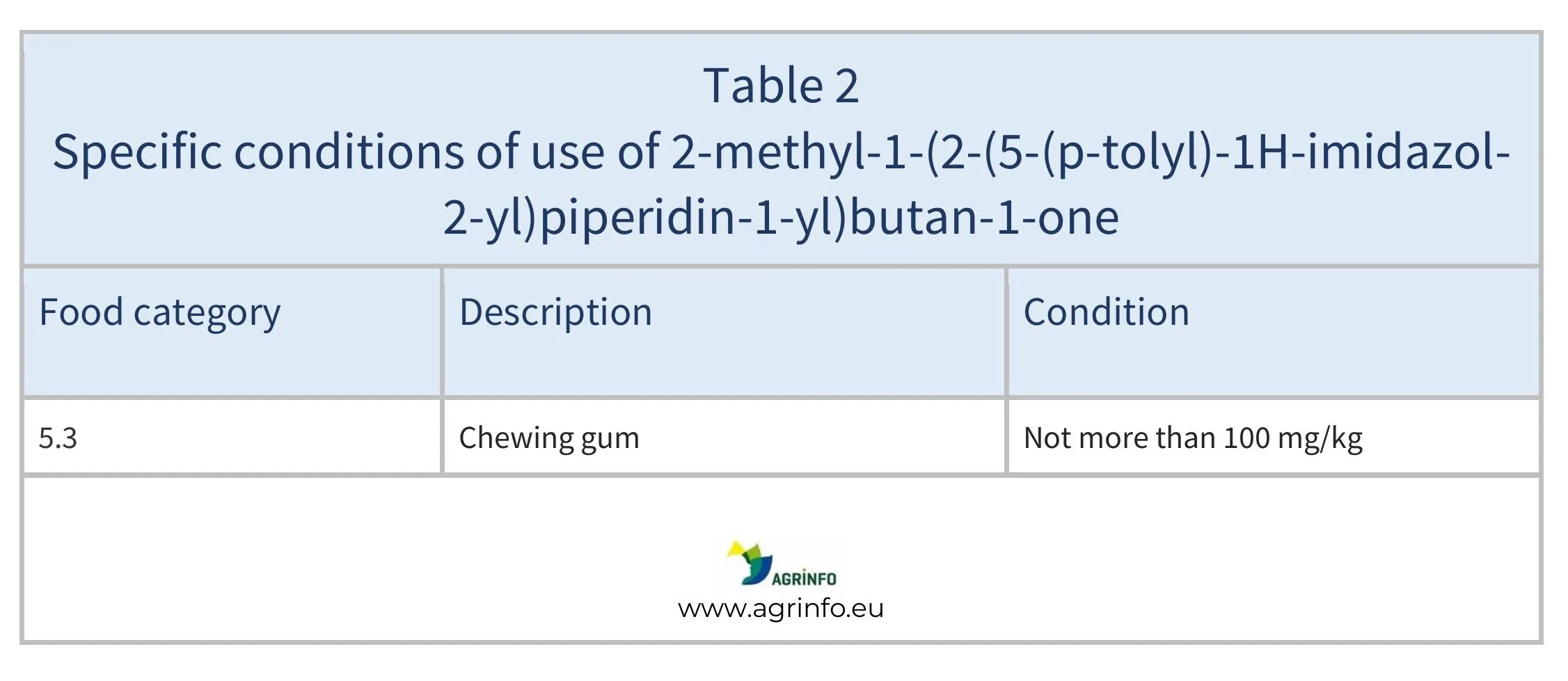
- 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one is only authorised to be used in chewing gum, as detailed in Table 2.
For more information on food categories, see the Guidance from the European Commission (2024).
Why?
The European Food Safety Authority (EFSA) evaluated the safety of Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one under specific conditions in certain foods, and concluded that there is no safety concern at the levels proposed by the applicant (EFSA 2024a, 2024b).
Timeline
Products in Tables 1 and 2 containing Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one, respectively, can be placed on the EU market from 25 June 2025.
Background
Regulation 1334/2008 prohibits adding certain undesirable natural substances to food. It also lays down maximum levels for some substances that are naturally present in flavourings, but that may raise concerns for human health. The Regulation defines different types of flavourings, and lists the substances for which evaluation and approval is required.
The Union list of flavouring substances approved for use in and on foods (Regulation 872/2012) was adopted in 2012.
When EFSA evaluates flavouring substances, it allocates them a unique identification number called an FL-number. FL comes from “FLAVIS”, the EU’s flavouring information system. The FL numbers for these substances are:
- Naringenin: 16.132
- 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one: 16.134.
The FL-number is not used for labelling purposes.
Resources
EFSA (2024a) Flavouring Group Evaluation 413 (FGE.413): Naringenin. EFSA Journal, 22(5): e8747.
EFSA (2024b) Flavouring group evaluation 419 (FGE.419): 2‐methyl‐1‐(2‐(5‐(p‐tolyl)‐1H‐imidazol‐2‐yl)piperidin‐1‐yl)butan‐1‐one. EFSA Journal, 22(5): e8750.
European Commission (2024) Guidance document describing the food categories in Part E of Annex II to Regulation (EC) No 1333/2008 on Food Additives.
Regulation 1334/2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods
Regulation 1331/2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings
Sources
Commission Regulation 2025/1112 as regards the inclusion of Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one in the Union list of flavourings
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU authorises flavourings Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1
Commission Regulation 2025/1112 as regards the inclusion of Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one in the Union list of flavourings
What is changing and why?
The European Union (EU) has approved the use of the flavourings Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one in certain types of food (see Tables 1 and 2 for details).
Timeline
Products in Tables 1 and 2 containing Naringenin and 2-methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one, respectively, can be placed on the EU market from 25 June 2025.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.