Food additives: glycerol (E 422, E 475, E 476)
- Food additives
- Food safety
Summary
The EU has set more stringent specifications concerning the use of several food additives: glycerol (E 422), polyglycerol esters of fatty acids (E 475), and polyglycerol polyricinoleate (E 476).
EU puts in place stricter specifications for glycerol food additives
Commission Regulation (EU) 2023/1329 of 29 June 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of polyglycerol polyricinoleate (E 476) and the Annex to Commission Regulation (EU) No 231/2012 as regards specifications for glycerol (E 422), polyglycerol esters of fatty acids (E 475) and polyglycerol polyricinoleate (E 476)
Update
The EU has set more stringent specifications concerning the use of several food additives: glycerol (E 422), polyglycerol esters of fatty acids (E 475), and polyglycerol polyricinoleate (E 476).
Impacted Products
processed products, edible ices, sauces
What is changing?
The European Commission has revised the specifications for the following food additives.
Glycerol (E 422)
- maximum limits for toxic elements including arsenic, lead, mercury and cadmium reduced
- identification method based on acrolein formation during heating and test for the presence of acrolein deleted
- maximum limit for acrolein included
- definition modified
Polyglycerol esters of fatty acids (E 475)
- maximum limits for toxic elements reduced
- maximum limits included for the sum of 3-monochloropropanediol (3-MCPD) and 3-MCPD fatty acid esters, glycidyl fatty acid esters, and erucic acid
- definition modified
Polyglycerol polyricinoleate (E 476)
- maximum limits for toxic elements reduced, including maximum limits for 3-MCPD and 3-MCPD fatty acid esters, and glycidyl fatty acid esters
- definition modified
- use in certain food categories modified (see Table 1).
Further details are set out in the Annexes of Regulation 2023/1329.
Why?
The changes are in response to the conclusions of recent scientific opinions on the safety and quality of these food additives (EFSA 2022a, 2022b, 2022c).
Timeline
The Regulation applies from 20 July 2023.
Foods containing these additives that were placed on the EU market before the Regulation entered into force, and that comply with the old rules, may remain on the market for 6 months even if they do not comply with the new rules.
What are the major implications for exporting countries?
The changes to the EU Regulation on authorised food additives may have an impact on countries exporting food products containing these additives to the EU market.
If exporting countries fail to comply with the new EU regulations, their products may be rejected at the EU border and prevented from entering the market. This could result in financial losses for exporters and a disruption of trade relations between the exporting country and the EU.
Recommended Actions
Suppliers of processed foods containing these additives may need to adjust their manufacturing practices and/or safety and quality controls to meet the new requirements.
Background
The proposed Regulation amends the Annex to Commission Regulation (EU) No 231/2012, and lays down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008.
Resources
EFSA (2022a) Follow‐up of the re‐evaluation of glycerol (E 422) as a food additive. EFSA Journal, 20(6): 7353.
EFSA (2022b) Follow‐up of the re‐evaluation of polyglycerol esters of fatty acids (E 475) as a food additive. EFSA Journal, 20(5): 7308.
EFSA (2022c) Follow‐up of the re‐evaluation of polyglycerol polyricinoleate (E 476) as a food additive. EFSA Journal, 20(5): 7294.
Sources
Draft Commission Regulation as regards specifications for glycerol (E 422), polyglycerol esters of fatty acids (E 475) and polyglycerol polyricinoleate (E 476)
PLAN/2022/2172, Annex
Tables & Figures
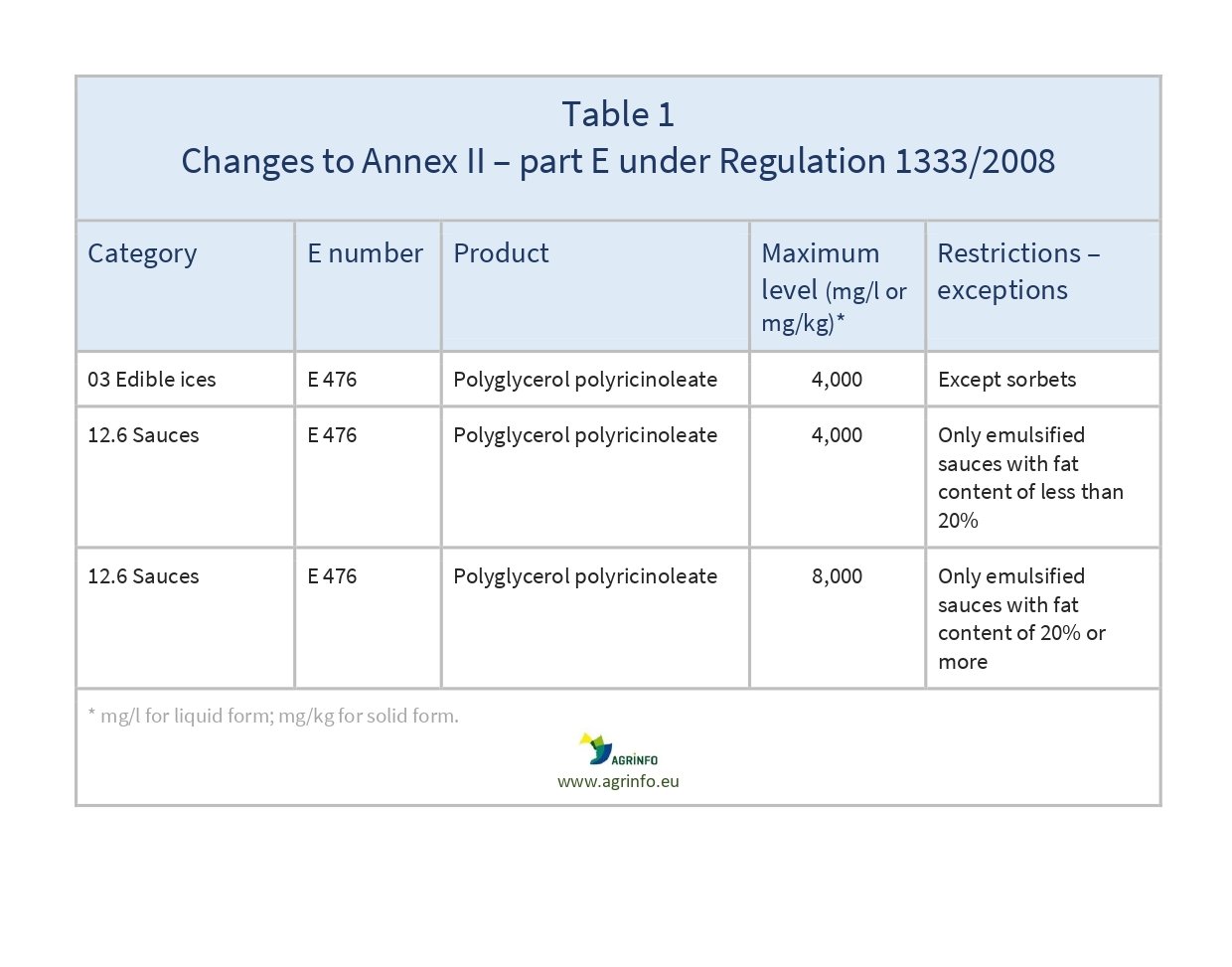
Source: Commission Regulation (EU) 2023/1329
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
