Latest pesticide non-renewals, withdrawals, restrictions, and non-approvals (2024)
- Food safety
- Pesticides
Summary
This report summarises recent EU decisions not to renew, or to withdraw, existing approvals for certain pesticide active substances. While these decisions primarily affect EU producers, they will probably be followed by legislation to reduce the maximum residue levels (MRLs) to 0.01 mg/kg or the limit of determination (LOD – the lowest level that can be detected using the most modern and reliable analytical methods). These decisions therefore provide an early indication of upcoming MRL changes, and the likely need to look for alternative solutions on crops for export to the EU.
This report also includes EU non-approval decisions. These are relevant to producers in exporting countries where these substances may be used locally. In such cases a default MRL of 0.01 mg/kg will be maintained.
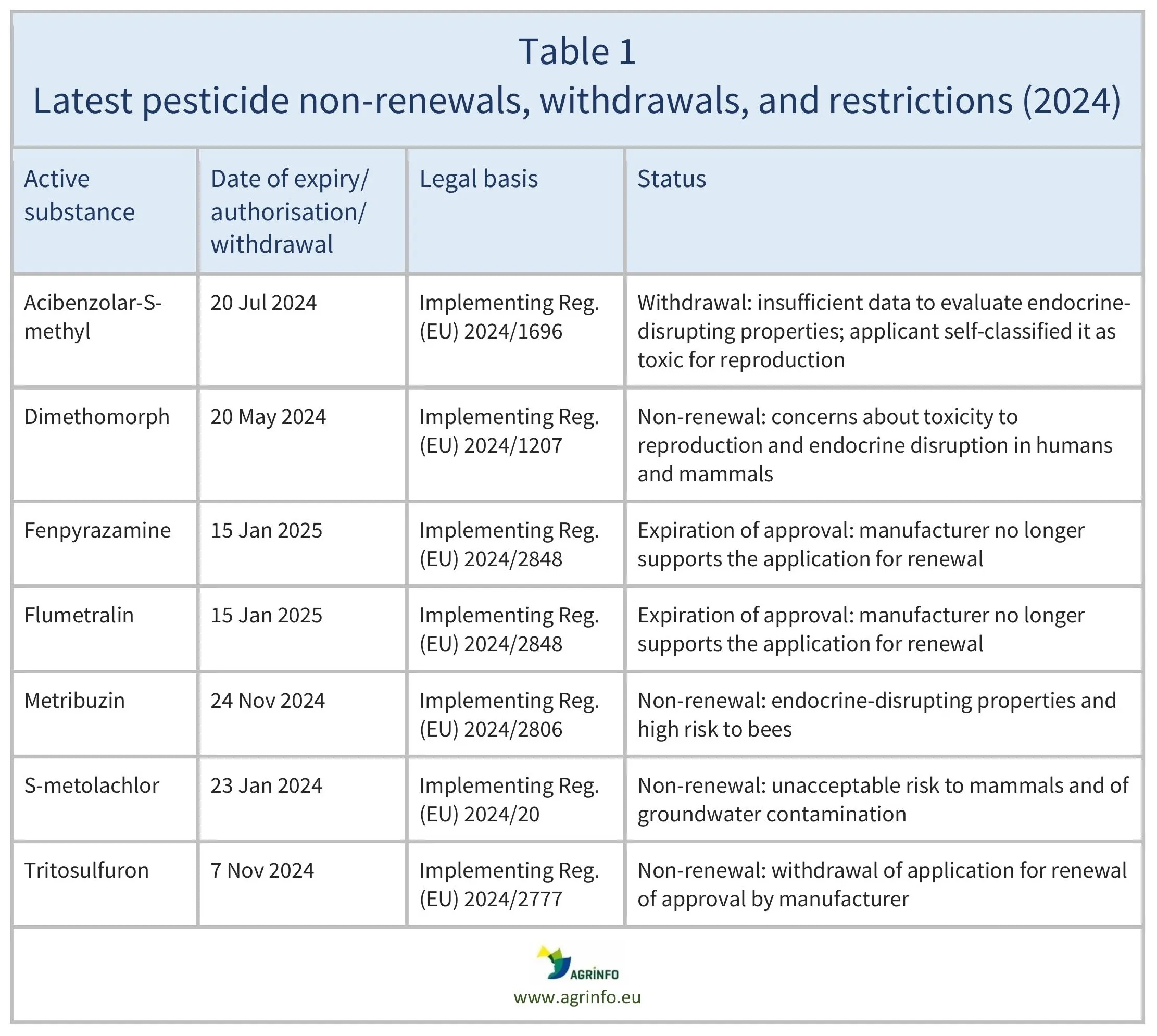
Non-renewals, withdrawals, restrictions, and non-approvals of pesticides for use in the EU introduced in 2024
Commission Implementing Regulations:
(EU) 2024/2848 as regards the approval periods of the active substances fenpyrazamine and flumetralin
(EU) 2024/2806 concerning the non-renewal of the approval of the active substance metribuzin
(EU) 2024/2777 concerning the non-renewal of approval of the active substance tritosulfuron
(EU) 2024/2766 concerning the non-approval of 1,3,7-trimethylxanthine (caffeine) as a basic substance
(EU) 2024/2197 concerning the non-approval of eggshell powder as a basic substance
(EU) 2024/1696 withdrawing the approval of the active substance acibenzolar-S-methyl
(EU) 2024/1207 concerning the non-renewal of the approval of the active substance dimethomorph
(EU) 2024/425 concerning the non-approval of the active substance asulam-sodium
(EU) 2024/20 concerning non-renewal of the approval of the active substance S-metolachlor
Update
This report summarises recent EU decisions not to renew, or to withdraw, existing approvals for certain pesticide active substances. While these decisions primarily affect EU producers, they will probably be followed by legislation to reduce the maximum residue levels (MRLs) to 0.01 mg/kg or the limit of determination (LOD – the lowest level that can be detected using the most modern and reliable analytical methods). These decisions therefore provide an early indication of upcoming MRL changes, and the likely need to look for alternative solutions on crops for export to the EU.
This report also includes EU non-approval decisions. These are relevant to producers in exporting countries where these substances may be used locally. In such cases a default MRL of 0.01 mg/kg will be maintained.
What is changing?
The EU systematically reviews the status of all pesticide active substances that are approved for use within the European Union. Recent non-renewals and withdrawals of approvals are summarised in Table 1. To check the expiry or review dates of other pesticide active substances, see the EU Pesticides Database.
In addition to the non-renewals and withdrawals in Table 1, the EU has recently decided not to approve 1,3,7-trimethylxanthine (caffeine) as a basic substance. Previously, the EU decided not to approve eggshell powder as a basic substance, as well as the active substance asulam-sodium.
Why?
EFSA (2023b) raised concerns about neurodevelopment effects associated with the use of eggshell powder due to its lead content, and therefore cannot consider its use as safe.
EFSA (2021a) identified endocrine-disrupting properties as the main hazard associated with the use of asulam-sodium. EFSA’s approval criteria were not satisfied and the applicant ultimately withdrew its application for the approval of this substance. This decision does not prevent the submission of a further application for approval of asulam-sodium.
Timeline
Expiry dates for the substances affected are listed in Table 1. After these dates, the pesticides can no longer be used in the EU.
The European Commission is expected to follow up with proposals to reduce or remove the MRLs for these substances, which will affect their use on crops for export to the EU. Any changes to MRLs are notified to the World Trade Organization Sanitary and Phytosanitary (WTO SPS) Committee, and details will be provided on the AGRINFO website.
What are the major implications for exporting countries?
Decisions not to renew or to withdraw EU approvals for the use of certain pesticide active substances mainly affect their use within the EU. However, following a non-renewal or withdrawal of approval, in most cases the EU starts the process of lowering or removing the associated MRLs. These are typically set at 0.01 mg/kg or the LOD. In many circumstances this means that they can no longer be used on crops for export to the EU, and action is needed.
Recommended Actions
Export sectors affected should start looking for alternative crop protection solutions to metribuzin, acibenzolar-S-methyl, and dimethomorph, or to assess possible adaptations of GAP. Exporting countries could also consider requesting EU import tolerances (for guidelines see European Commission 2021). See Pesticide residue import tolerance MRLs explained.
Background
For decisions taken in 2023, see Latest pesticide non-renewals, withdrawals, and restrictions (2023).
Pesticide active substances are approved for up to a maximum period of 15 years. Manufacturers may apply for reapproval of a substance for a period not more than 15 years. The EU systematically reviews all active substances.
Substances can be:
- not reapproved: if there is insufficient data to permit reapproval, or because the manufacturer does not seek reapproval
- withdrawn: where specific consumer health or environmental issues are identified, sometimes before the normal expiry date
- restricted: where data supports renewal, but only under new specific conditions of use.
Where an authorisation for an active substance is withdrawn or expires due to withdrawal of approval or non-renewal, the European Commission will prepare a draft measure to delete the relevant existing MRLs. In practice, the Commission starts this procedure once all existing authorisations for that active substance have been revoked. MRLs are set either to a default value of 0.01 mg/kg, or to the lowest limit technically possible using current analytical methods. MRLs based on the Codex MRLs (CXLs) are not deleted where there is no risk to EU consumers, or no global environmental concern. Changes to MRLs that impact trade are always notified to the WTO SPS Committee.
The precise timing of changes to MRLs resulting from the withdrawal or non-renewal of active substances is difficult to predict. In its review of pesticide policy, the Commission committed to “enhance communication efforts on the impacts of the PPP Regulation on MRLs as well as the timing of the various procedures to make the EU system more predictable for non-EU countries, including for the cut-off criteria” (European Commission 2020).
Import tolerances can be requested in anticipation of potential changes to MRLs (see Overview table 2009–2020), but applicants must demonstrate the existence of relevant good agricultural practices (GAP) in the country of origin, and the safety of the proposed MRLs. Guidelines are available on the requirements and process for establishing MRLs and import tolerances (European Commission 2021).
Resources
Bryant Christie Inc. & CropLife International: EU Pesticide Renewal Monitors [select Topic = Renewal Monitor].
EFSA (2021a) Updated peer review of the pesticide risk assessment of the active substance asulam (variant evaluated asulam‐sodium). EFSA Journal, 19(11): 6921.
EFSA (2021b) Peer review of the pesticide risk assessment for the active substance acibenzolar‐S‐methyl in light of confirmatory data submitted. EFSA Journal, 19(7): 6687.
EFSA (2023a) Peer review of the pesticide risk assessment of the active substance dimethomorph. EFSA Journal, 21(6): 8032.
EFSA (2023b) Overall conclusions on the application for approval of eggshell powder as a basic substance to be used in plant protection as a fungifuge on grapevines. EFSA supporting publication, 20(11): EN-8434.
EFSA (2023c) Peer review of the pesticide risk assessment of the active substance metribuzin. EFSA Journal, 21(8): 8140.
European Commission: Overview table 2009–2020.
European Commission (2020) Evaluation of Regulation (EC) No 1107/2009 on the placing of plant protection products on the market and of Regulation (EC) No 396/2005 on maximum residue levels of pesticides.
European Commission (2021) Technical Guidelines: MRL Setting Procedure in Accordance with Article 6 to 11 of Regulation (EC) No 396/2005 and Article 8 of Regulation (EC) No 107/2009.
ITC, UN, and WTO: ePing SPS & TBT Platform
Sources
Commission Implementing Regulations:
- 2024/20 concerning non-renewal of the approval of the active substance S-metolachlor
- 2024/425 concerning non-approval of the active substance asulam-sodium
- 2024/1207 concerning the non-renewal of the approval of the active substance dimethomorph
- 2024/1696 withdrawing the approval of the active substance acibenzolar-S-methyl
- 2024/2197 concerning the non-approval of eggshell powder as a basic substance
- 2024/2766 concerning the non-approval of 1,3,7-trimethylxanthine (caffeine) as a basic substance
- 2024/2777 concerning the non-renewal of approval of the active substance tritosulfuron
- 2024/2806 concerning the non-renewal of the approval of the active substance metribuzin
- 2024/2848 as regards the approval periods of the active substances fenpyrazamine and flumetralin
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
Non-renewals, withdrawals, restrictions, and non-approvals of pesticides for use in the EU introduced in 2024
Commission Implementing Regulations:
- 2024/20 concerning non-renewal of the approval of the active substance S-metolachlor
- 2024/425 concerning non-approval of the active substance asulam-sodium
- 2024/1207 concerning the non-renewal of the approval of the active substance dimethomorph
- 2024/1696 withdrawing the approval of the active substance acibenzolar-S-methyl
- 2024/2197 concerning the non-approval of eggshell powder as a basic substance
- 2024/2766 concerning the non-approval of 1,3,7-trimethylxanthine (caffeine) as a basic substance
- 2024/2777 concerning the non-renewal of approval of the active substance tritosulfuron
- 2024/2806 concerning the non-renewal of the approval of the active substance metribuzin
- 2024/2848 as regards the approval periods of the active substances fenpyrazamine and flumetralin
What is changing and why?
The EU systematically reviews the status of all pesticide active substances that are approved for use within the European Union. Recent withdrawals of approvals are summarised in Table 1.
In addition to the withdrawals and non-renewals in Table 1, the EU has recently decided not to approve 1,3,7-trimethylxanthine (caffeine) as a basic substance. Previously the EU decided not to approve eggshell powder as a basic substance due to concerns about neurodevelopment effects associated with its lead content, and eggshell powder as a basic substance due to concerns about neurodevelopment effects associated with its lead content. Previously the EU decided not to approve the active substance asulam-sodium because the approval criteria of the European Food Safety Authority (EFSA) could not be satisfied, and the applicant ultimately withdrew its application. These decisions do not prevent the submission of a further application for approval of these active substances.
Actions
Export sectors affected should start looking for alternative crop protection solutions to metribuzin, acibenzolar-S-methyl, and dimethomorph, or assess possible adaptations of good agricultural practices (GAP). Exporting countries can also request EU import tolerances (for guidelines see European Commission 2021). See Pesticide residue import tolerance MRLs explained.
Timeline
The pesticides listed in Table 1 can no longer be used in the EU after the expiry dates shown.
For each substance, the European Commission is expected to follow up with proposals to reduce or remove the MRLs. Suppliers in non-EU countries may need to adjust agricultural practices to meet reduced MRLs. Any changes to MRLs are notified to the World Trade Organization Sanitary and Phytosanitary (WTO SPS) Committee. Details will be provided on the AGRINFO website.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.