Maximum levels for 3-MCPD in infant formulae
- Contaminants
- Food safety
Summary
The EU has reduced the maximum levels of the sum of 3-monochlorpropanediol (3-MCPD) and 3-MCPD fatty acid esters in certain food products for infants and young children.
EU reduces 3-MCPD maximum levels in infant formula
Commission Regulation (EU) 2024/1003 of 4 April 2024 amending Regulation (EU) 2023/915 as regards maximum levels for the sum of 3-monochlorpropanediol (3-MCPD) and 3-MCPD fatty acid esters in infant formulae, follow-on formulae and food for special medical purposes intended for infants and young children and young child formulae
Update
The EU has reduced the maximum levels of the sum of 3-monochlorpropanediol (3-MCPD) and 3-MCPD fatty acid esters in certain food products for infants and young children.
Impacted Products
Infant formulae, follow-on formulae, food for special medical purposes intended for infants and young children, young child formulae
What is changing?
The European Commission has reduced the maximum levels of the sum of 3-MCPD and 3-MCPD fatty acid esters in infant formulae, follow-on formulae, food for special medical purposes intended for infants and young children, and young child formulae. The changes are shown in Table 1.
Why?
EFSA (2018) expressed health concerns about the presence of these substances, especially in foods for infants and young children.
Timeline
The new maximum levels will apply from 1 January 2025.
Recommended Actions
Non-EU suppliers of these products for infants should evaluate current levels of 3-MCPD and its esters to check compliance with this Regulation, and consider strategies for reducing the presence of this contaminant.
Background
3-MCPD and its esters are contaminants that can be formed during food processing, particularly when high temperatures are applied to fats and oils.
The original maximum levels for 3-MCPD and its esters in specific food products for infants and young children were established by Regulation (EU) 2020/1322.
The EU aims to set maximum levels following the principle that they should be as low as reasonably achievable when applying good practices, and on the basis of scientific advice provided by the European Food Safety Authority (EFSA), taking into account data on the occurrence of contaminants in foodstuffs from various origins. See EU legislation on contaminants – maximum levels explained.
Resources
EFSA (2018) Update of the risk assessment on 3-monochloropropane diol and its fatty acid esters. EFSA Journal, 16(1): 5083.
European Commission (2008) Factsheet: Food contaminants.
Regulation (EU) 2020/1322 as regards maximum levels of 3‐monochloropropanediol (3-MCPD), 3-MCPD fatty acid esters and glycidyl fatty acid esters in certain foods
Regulation (EU) 2023/915 on maximum levels for certain contaminants in food
Regulation (EEC) No 315/93 laying down Community procedures for contaminants in food
Sources
Regulation 2024/1003 as regards maximum levels for the sum of 3-monochlorpropanediol (3-MCPD) and 3-MCPD fatty acid esters in infant formulae, follow-on formulae and food for special medical purposes
Tables & Figures
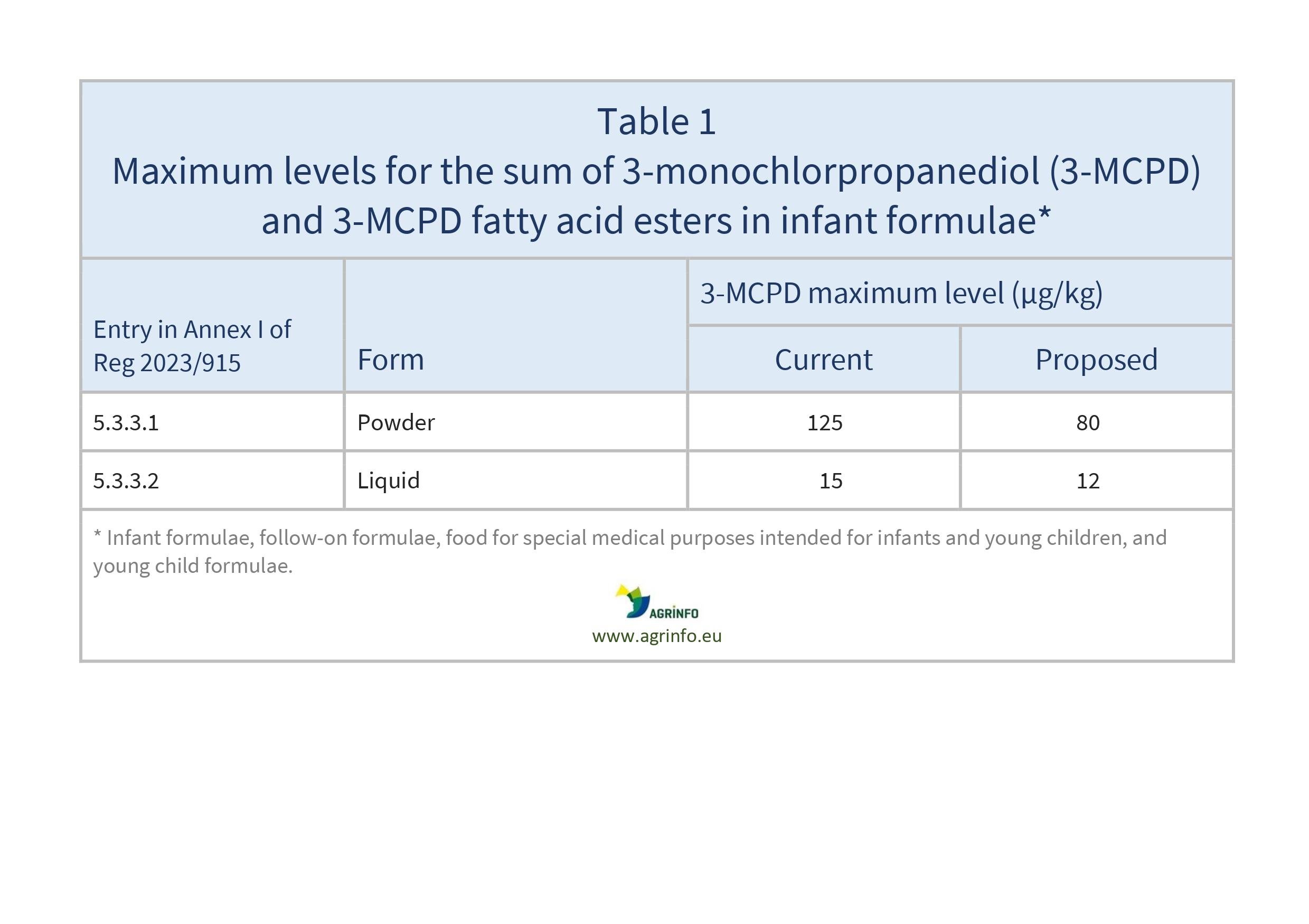
Source: Regulation (EU) 2023/915
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU reduces 3-MCPD maximum levels in infant formula
Regulation 2024/1003 on maximum levels for the sum of 3-monochlorpropanediol (3-MCPD) and its fatty acid esters in infant formulae, follow-on formulae and food for special medical purposes
What is changing and why?
The European Commission has reduced the maximum levels for the sum of 3-MCPD and 3-MCPD fatty acid esters in infant formulae, follow-on formulae, and food for special medical purposes. The changes are shown in Table 1.
This is because the European Food Safety Authority has expressed health concerns about the presence of these substances in foods, especially in foods for infants and young children.
Actions
Non-EU suppliers of these products for infants should evaluate current levels of 3-MCPD and its esters to check compliance with this Regulation, and consider strategies for reducing the presence of this contaminant.
Timeline
Applies from 1 January 2025.
Tables & Figures

Source: Regulation (EU) 2023/915
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
