Maximum levels for T-2 and HT-2 toxins in foods
- Contaminants
- Food safety
Summary
The EU has established maximum levels for the sum of T-2 and HT-2 toxins in cereals and cereal products intended for human consumption.
EU establishes maximum levels for T-2 and HT-2 toxins in cereal-based foods
Commission Regulation (EU) 2024/1038 of 9 April 2024 amending Regulation (EU) 2023/915 as regards maximum levels of T-2 and HT-2 toxins in food
Update
The EU has established maximum levels for the sum of T-2 and HT-2 toxins in cereals and cereal products intended for human consumption.
Impacted Products
Unprocessed cereal grains, barley, maize, oats, milling products of cereals, bran, maize products, bakery wares, pasta, cereal snacks, breakfast cereals, baby food, food for special medical purposes intended for infants and young children
What is changing?
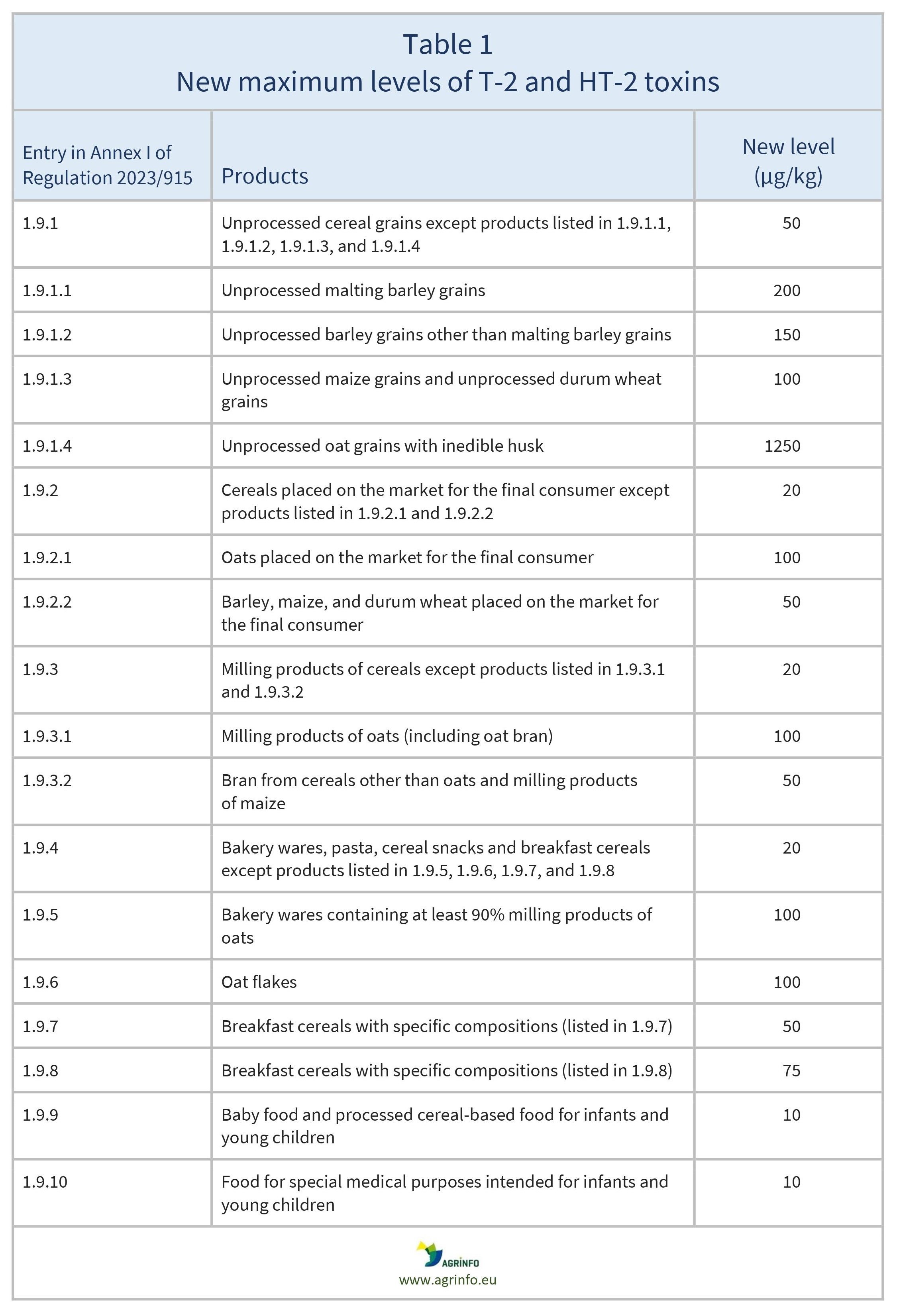
The EU has introduced maximum levels for T-2 and HT-2 in various foods. The new maximum levels are highlighted in Table 1.
In addition, the EU proposes to monitor and report the presence of T-2 and HT-2 toxins in oats with a view to potentially reducing maximum levels further in the future.
Why?
An evaluation of T-2 and HT-2 toxins in food by the European Food Safety Authority (EFSA 2011) identified potential health risks, especially to children. To ensure high public health protection levels, the EU concluded that maximum levels should be set for certain foods, based on the data submitted to EFSA.
Oat grains show particularly high levels of these toxins and require further monitoring.
Timeline
The new maximum levels apply from 1 July 2024.
Recommended Actions
Non-EU suppliers of cereal products should urgently evaluate current levels of T-2 and HT-2 toxins in these products to identify any potential non-compliance and strategies for reducing the presence of these toxins.
Agricultural practices to reduce the risk of contamination by T-2 and HT-2 toxins include crop rotation and selecting resistant crop varieties. EU recommendations on the presence of T-2 and HT-2 toxins in cereals and cereal products can be found in Commission Recommendation 2013/165/EU.
More generally, to mitigate the risks of potential non-compliance with maximum levels on contaminants, non-EU countries should:
- ensure sampling and testing capacity for those contaminants listed in EU Regulations; training is available, for example through the European Commission’s Better Training for Safer Food (BTSF) Academy
- ensure that, where feasible, established strategies for reducing contamination are systematically disseminated and implemented in relevant agricultural value chains
- contribute data to EFSA’s annual data collection process to ensure that risk assessments undertaken by EFSA have a complete picture of the current prevalence of contaminants in non-EU countries.
Background
Mycotoxins are secondary metabolites produced by fungi that grow naturally in food and feedstuffs including grains, nuts, and fruits, particularly under warm and humid conditions. T-2 and HT-2 toxins are specific types of mycotoxin.
The European Union establishes maximum allowable limits for mycotoxins in food products to ensure that they remain within safe consumption levels. The legal framework for these maximum levels is established by Council Regulation (EEC) 315/93 (basic principles) and Commission Regulation (EU) 2023/915 (maximum levels). The EU aims to set maximum levels following the principle that they should be as low as reasonably achievable by applying good practices, and on the basis of scientific advice provided by EFSA, taking into account data on the occurrence of contaminants in foodstuffs from various origins. See EU legislation on contaminants – maximum levels explained.
Resources
EFSA (2011) Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA Journal 9(12): 2481.
European Commission (2008) Factsheet: Food contaminants.
The EU has issued a series of Guidance Documents on the measurement and control of contaminants, including a Guidance document on identification of mycotoxins and plant toxins in food and feed.
Commission Regulation (EU) 2023/915
Council Regulation (EEC) No 315/93
Sources
Commission Regulation (EU) 2024/1038 of 9 April 2024 amending Regulation (EU) 2023/915 as regards maximum levels of T-2 and HT-2 toxins in food
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU establishes maximum levels for T-2 and HT-2 toxins in cereal-based foods
Commission Regulation (EU) 2024/1038 as regards maximum levels of T-2 and HT-2 toxins in food
What is changing and why?
The EU has introduced maximum levels for T-2 and HT-2 in various foods. The new maximum levels are highlighted in Table 1.
This is because an evaluation by the European Food Safety Authority identified potential health risks, especially to children.
Oat grains show particularly high levels of these toxins and will require further monitoring.
Actions
Non-EU suppliers of cereal products should urgently evaluate current levels of T-2 and HT-2 toxins in these products to identify any potential non-compliance and strategies for reducing the presence of these toxins.
Agricultural practices to reduce the risk of contamination by T-2 and HT-2 toxins include crop rotation and selecting resistant crop varieties.
EU recommendations on the presence of T-2 and HT-2 toxins in cereals and cereal products can be found in Commission Recommendation 2013/165/EU.
Timeline
Date of entry into force: 1 July 2024.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.