Maximum levels of delta-9-tetrahydrocannabinol (Δ9-THC) in hemp seeds
- Contaminants
Summary
Regulation 2023/915 sets maximum levels for delta-9-tetrahydrocannabinol (∆9-THC) authorised in hemp seeds and derived products sold on the European Union (EU) market.
Maximum levels for delta-9-tetrahydrocannabinol (∆9-THC) in hemp seeds and derived products
Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006
Update
Regulation 2023/915 sets maximum levels for delta-9-tetrahydrocannabinol (∆9-THC) authorised in hemp seeds and derived products sold on the European Union (EU) market.
Impacted Products
Hemp seeds and derived products
What is changing?
The maximum levels are presented in Table 1.
Why?
In 2015, the European Food Safety Authority assessed the risk to human health related to the presence of Δ9-THC in milk and dairy products (EFSA 2015).
EFSA (2020) published a scientific report assessing exposure to Δ9-THC, and found that the rate of 1 µg Δ9-THC per kg body weight as an acute reference dose was exceeded in certain exposure estimates. The European Commission therefore considers that maximum levels should be set for hemp seeds and derived products.
Timeline
These maximum levels for delta-9-tetrahydrocannabinol (∆9-THC) apply from 1 January 2023.
Recommended Actions
Exporters of hemp seeds and derived products must ensure that adequate measures are put in place to prevent contamination during processing in order to meet the newly established levels (BfR 2018).
Resources
BfR (2018) Tetrahydrocannabinol levels are too high in many hemp-containing foods - health impairments are possible. Bundesinstitut für Risi.
EFSA (2015) Scientific Opinion on the risks for human health related to the presence of tetrahydrocannabinol (THC) in milk and other food of animal origin. EFSA Journal, 13(6): 4141.
EFSA (2020) Acute human exposure assessment to tetrahydrocannabinol (Δ9‐THC). EFSA Journal, 18(1): 5953.
Sources
Commission Regulation (EU) 2023/915 on maximum levels for certain contaminants in food
Tables & Figures
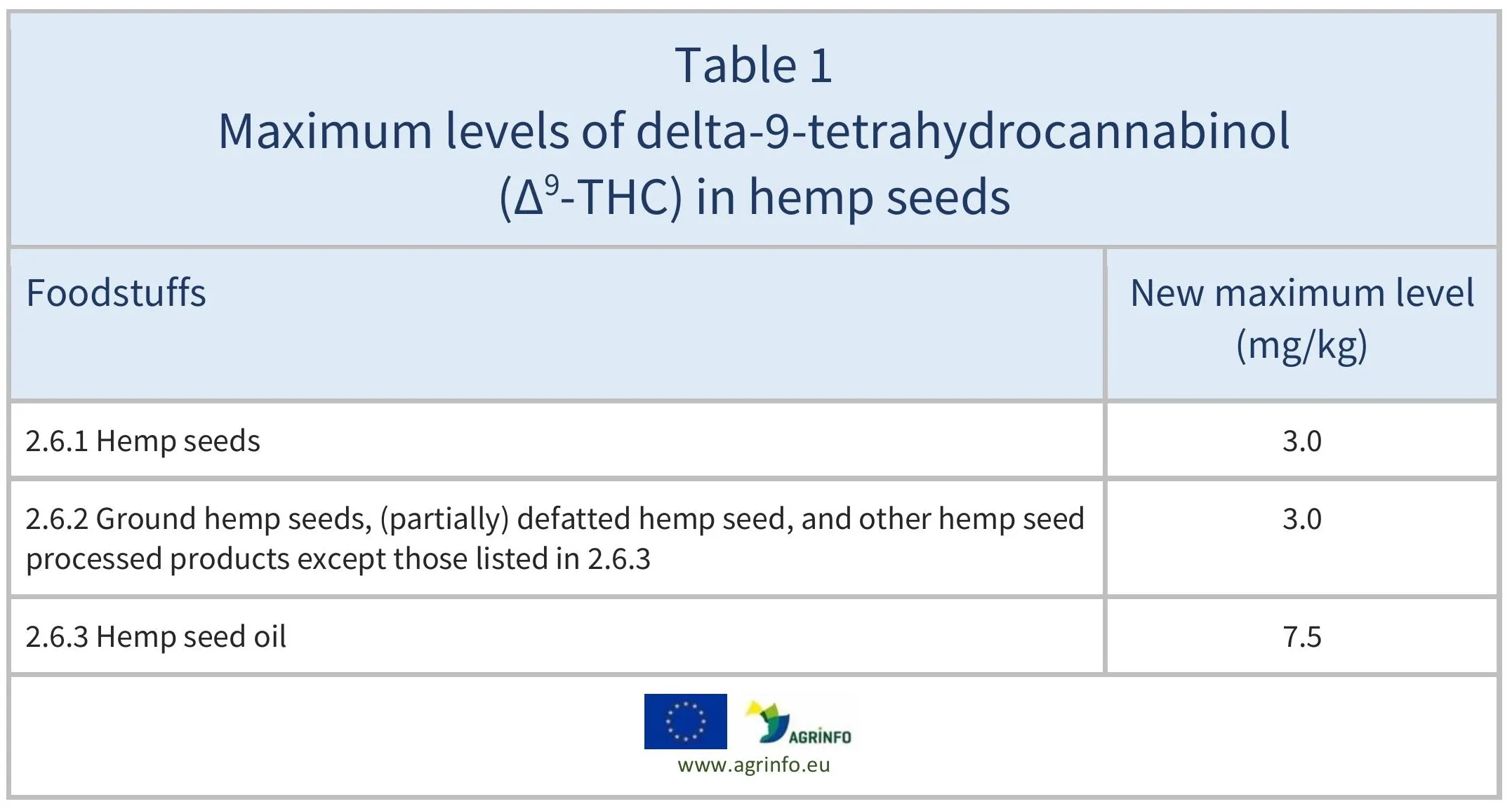
Source: Regulation 2023/915
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
Maximum levels for delta-9-tetrahydrocannabinol (∆9-THC) in hemp seeds and derived products
Commission Regulation (EU) 2023/915 on maximum levels for certain contaminants in food
What is changing and why?
The European Union has set maximum levels of delta-9-tetrahydrocannabinol (∆9-THC) for hemp seeds and derived products (see Table 1). This is to avoid potential health risks due to the presence of ∆9-THC in milk and dairy products.
Timeline
These maximum levels for delta-9-tetrahydrocannabinol (∆9-THC) apply from 1 January 2023.
Tables & Figures

Source: Regulation 2023/915
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
