Maximum residue levels: benzovindiflupyr, quinclorac, spiromesifenin
- Food safety
- Pesticide MRLs
Summary
The EU has raised the maximum residue levels (MRLs) for benzovindiflupyr, quinclorac, and spiromesifenin on various products to bring them into alignment with established Codex MRLs.
EU aligns MRLs for benzovindiflupyr, quinclorac, and spiromesifenin on various products with Codex standards
Commission Regulation (EU) 2024/1355 of 21 May 2024 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for benzovindiflupyr, chlorantraniliprole, emamectin, quinclorac, spiromesifen, and triflumuron in or on certain products
Update
The EU has raised the maximum residue levels (MRLs) for benzovindiflupyr, quinclorac, and spiromesifenin on various products to bring them into alignment with established Codex MRLs.
Impacted Products
Ginseng, cranberries, rapeseeds, oranges, mangoes, beans without pods
What is changing?
The EU has aligned the MRLs with Codex MRLs for the following substances and products:
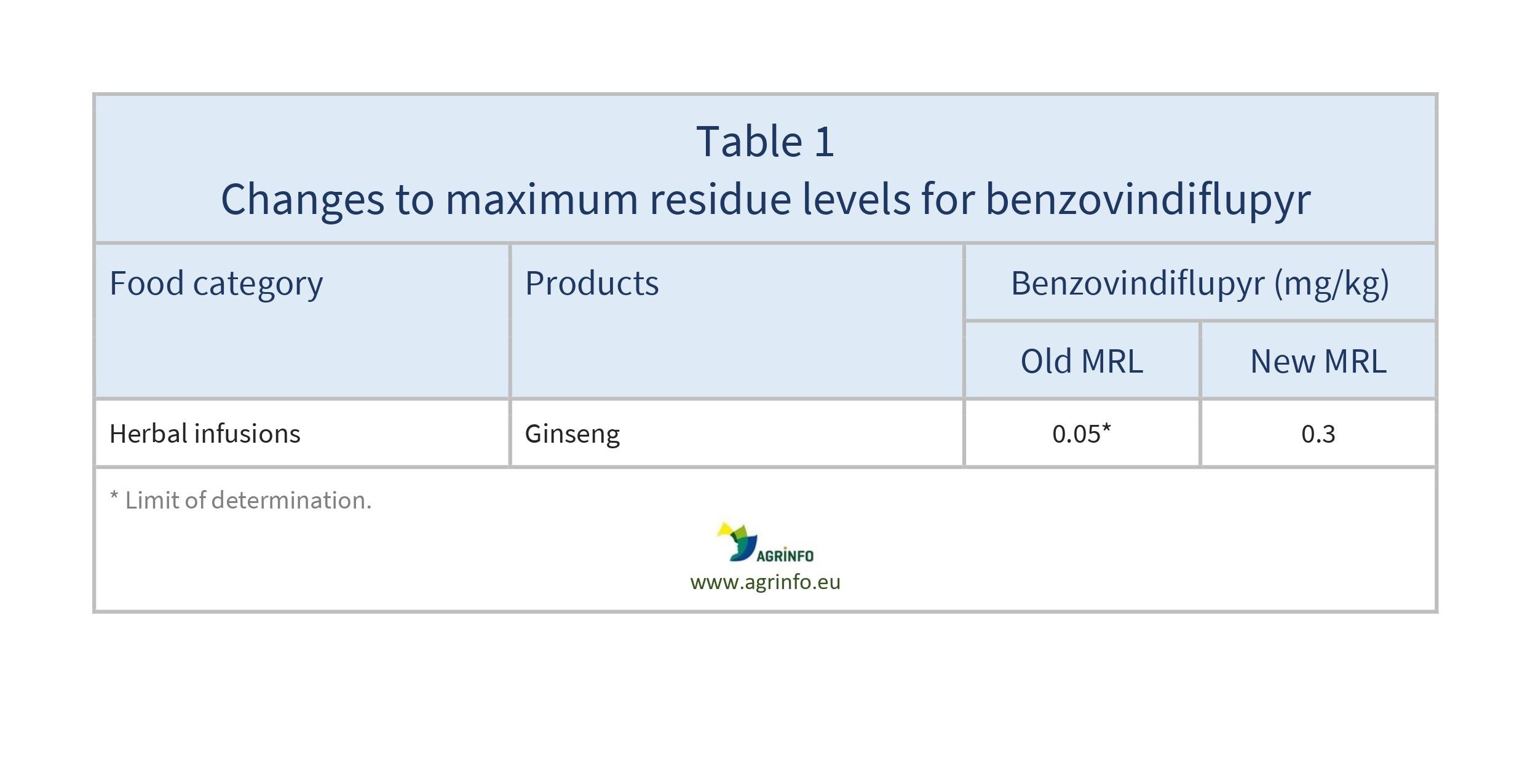
- benzovindiflupyr: ginseng (see Table 1)
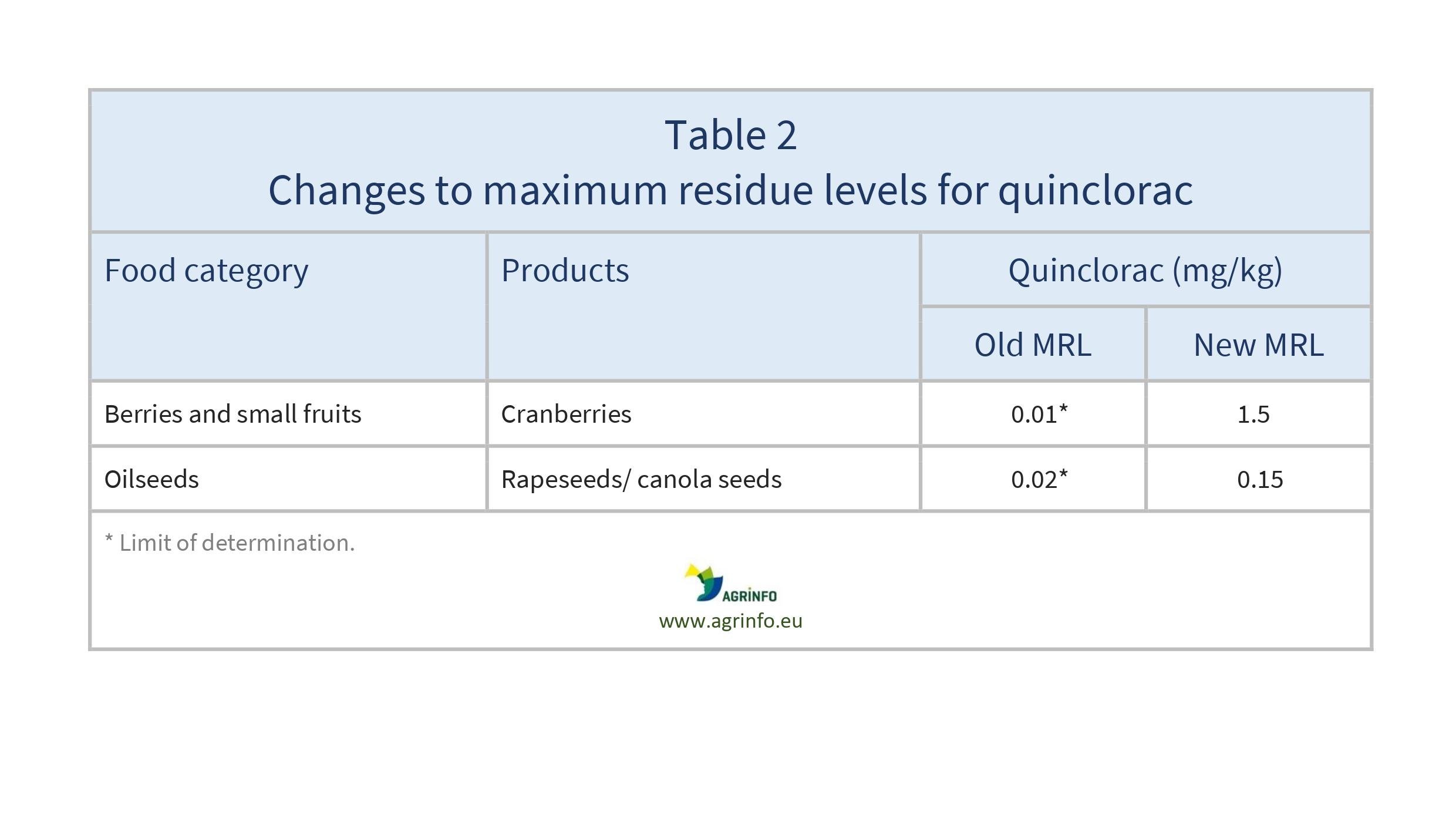
- quinclorac: cranberry and rapeseeds (see Table 2)
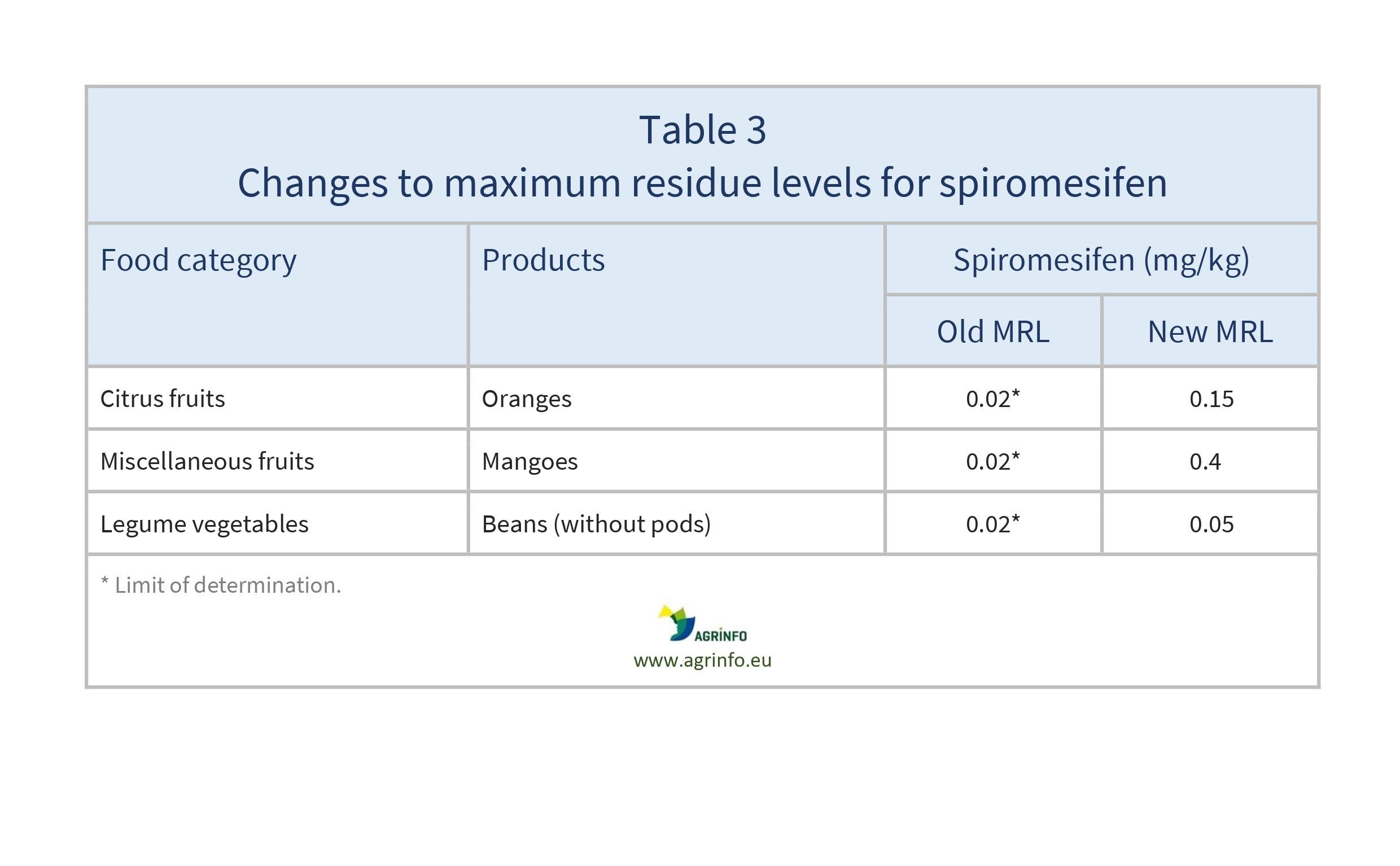
- spiromesifen: oranges, mangoes, and beans without pods (see Table 3).
Why?
The European Food Safety Authority (EFSA 2023) did not identify any health risks to consumers for Codex MRLs (CXLs) set for these substance/product combinations.
Timeline
The new MRLs apply from 11 June 2024.
Background
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
EFSA (2023) Scientific support for preparing an EU position in the 54th Session of the Codex Committee on Pesticide Residues (CCPR). EFSA Journal, 21(8): 1–303.
Sources
Commission Regulation (EU) 2024/1355 as regards maximum residue levels for benzovindiflupyr, chlorantraniliprole, emamectin, quinclorac, spiromesifen, and triflumuron in or on certain products
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU aligns MRLs for benzovindiflupyr, quinclorac, and spiromesifenin on various products with Codex standards
Commission Regulation (EU) 2024/1355 as regards maximum residue levels for benzovindiflupyr, chlorantraniliprole, emamectin, quinclorac, spiromesifen, and triflumuron in or on certain products
What is changing and why?
The EU has adopted Codex MRLs for the following substances and products:
- benzovindiflupyr: ginseng (see Table 1)
- quinclorac: cranberry and rapeseeds (see Table 2)
- spiromesifen: oranges, mangoes and beans without pods (see Table 3).
The European Food Safety Authority (EFSA) considers that these levels present no health risks for consumers of these products.
Timeline
The new MRLs apply from 11 June 2024.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.