Maximum residue levels for acetamiprid
- Pesticide MRLs
Summary
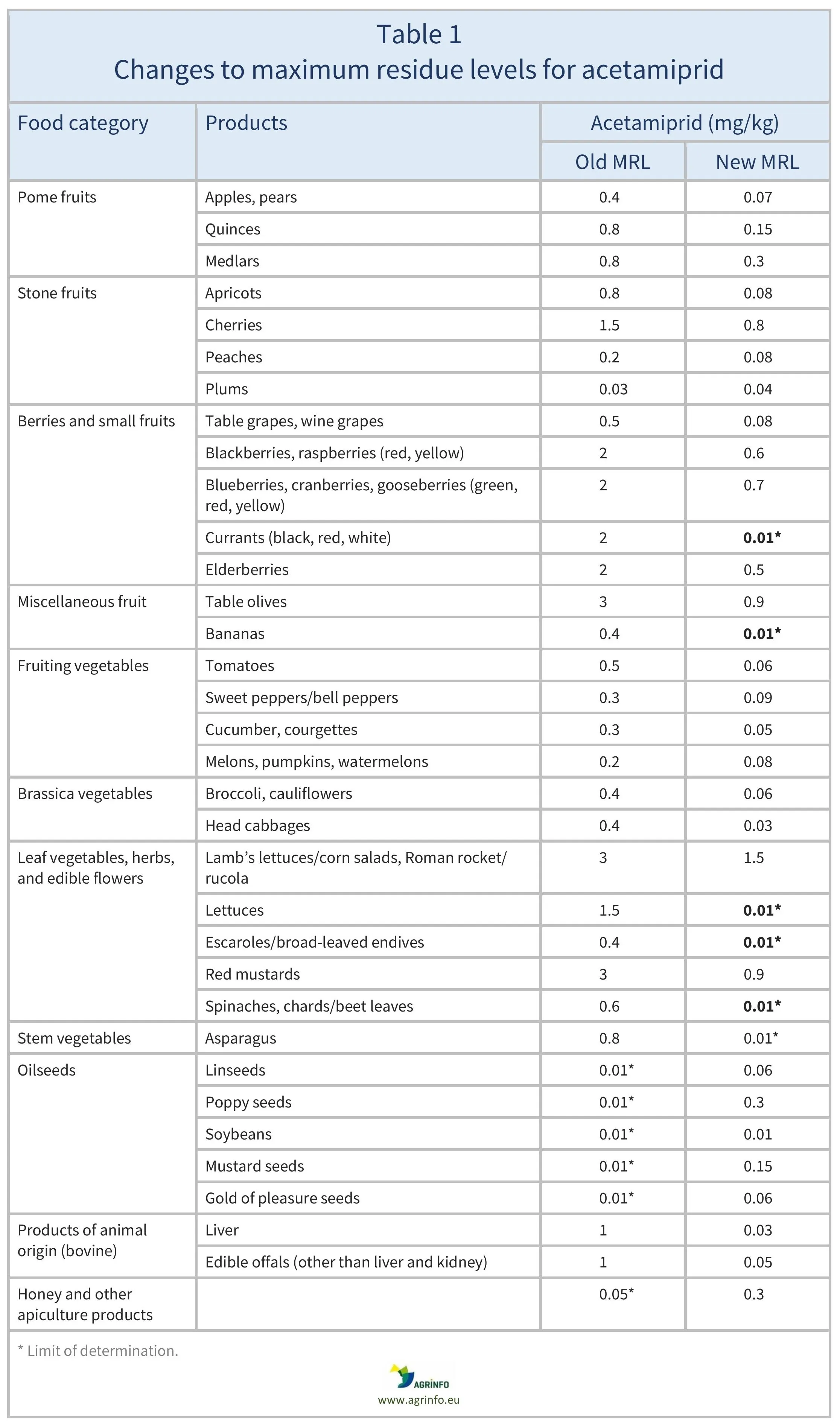
The European Union (EU) is again increasing the maximum residue level (MRL) for acetamiprid on honey and other apiculture products: from 0.3 to 1 mg/kg. This follows MRL increases for acetamiprid in June 2025 on a range of products including honey.
In January 2025, the MRLs for acetamiprid were reduced to the limit of determination (LOD) on many other products including asparagus, bananas, chards, currants, escaroles, lettuces, and spinach – see Table 1 for details. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.)
EU raises acetamiprid MRL for honey
Draft Commission Regulation amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for acetamiprid, aclonifen, deltamethrin, oxathiapiprolin and potassium phosphonates in or on certain products
Draft Annex II
Update
The European Union (EU) is again increasing the maximum residue level (MRL) for acetamiprid on honey and other apiculture products: from 0.3 to 1 mg/kg. This follows MRL increases for acetamiprid in June 2025 on a range of products including honey.
In January 2025, the MRLs for acetamiprid were reduced to the limit of determination (LOD) on many other products including asparagus, bananas, chards, currants, escaroles, lettuces, and spinach – see Table 1 for details. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.)
Impacted Products
Apples, pears, quinces, medlars, apricots, cherries, peaches, plums, table grapes, wine grapes, blackberries, raspberries (red, yellow), blueberries, cranberries, gooseberries (green, red, yellow), currants (black, red, white), elderberries, table olives, bananas, tomatoes, sweet peppers/bell peppers, cucumbers, courgettes, melons, pumpkins, watermelons, broccoli, cauliflowers, head cabbages, lamb’s lettuces/corn salads, Roman rocket/ rucola, lettuces, escaroles/broad-leaved endives, red mustards, spinaches, chards/beet leaves, asparagus, poppy seeds, mustard seeds, gold of pleasure seeds, soyabeans, bovine liver, bovine edible offals (other than liver and kidney), honey
What is changing?
The MRLs for acetamiprid were amended in January and June 2025, as summarised in Table 1. The EU is now increasing the MRL for acetamiprid on honey and other apiculture products from 0.3 to 1 mg/kg.
Why?
A modification of the existing MRLs was requested for honey and other apiculture products. The European Food Safety Authority (EFSA 2025) has concluded that the new MRL proposed for these products is acceptable with regard to consumer safety.
Following a review of the toxicological properties and MRLs for acetamiprid, EFSA (2024a) identified a lower acceptable daily intake (ADI) and a lower acute reference dose (ARfD). For products where the existing MRLs caused the new ARfD to be exceeded, EFSA suggested lower MRLs that pose no health risks for the consumer.
EFSA evaluated requests for MRL modifications on plums, linseeds, poppy seeds, mustard seeds, gold of pleasure seeds, and honey, and concluded that they were acceptable with regard to consumer safety (EFSA 2022). In 2024, Codex Alimentarius adopted a maximum residue limit (CXL) of 0.01 mg/kg for soyabeans, which EFSA reviewed and concluded to be safe for consumers (EFSA 2024b).
Timeline
The new MRL for honey and other apiculture products is expected to apply from February 2026.
The MRLs adopted under Regulation 2025/158 apply from 19 August 2025. Suppliers that exported products before 19 August 2025 must ensure that they comply with the new MRLs when they remain on the market.
The MRLs adopted under Regulation 2025/1212 apply from 20 August 2025.
Recommended Actions
Suppliers to the EU market of asparagus, bananas, chards, currants, escaroles, lettuces, and spinach should review their current use of acetamiprid and seek alternative solutions. Suppliers of other affected products should review their use of acetamiprid and assess whether any changes will be needed to existing good agricultural practices (GAP).
A new review of this substance will be carried out in February 2027. Suppliers should continue to monitor levels of acetamiprid and the GAP for its use in apples, pears, quinces, apricots, sweet peppers, cucumbers, and courgettes, so that data can be submitted to the European Commission before 24 September 2026.
Background
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
EFSA (2022) Modification of the existing maximum residue levels for acetamiprid in honey and various oilseed crops. EFSA Journal, 20(8): 7535.
EFSA (2024a) Statement on the toxicological properties and maximum residue levels of acetamiprid and its metabolites. EFSA Journal, 22(5): e8759.
EFSA (2024b) Scientific support for preparing an EU position in the 55th Session of the Codex Committee on Pesticide Residues (CCPR). EFSA Journal, 22(7): e8841.
EFSA (2025) Modification of the existing maximum residue level for acetamiprid in honey. EFSA Journal, 23(3): e9300.
Commission Regulation (EU) 2025/1212 as regards maximum residue levels for acetamiprid in or on certain products
Commission Regulation (EU) 2025/158 as regards maximum residue levels for acetamiprid in or on certain products
Sources
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU raises acetamiprid MRL for honey
Draft Commission Regulation as regards maximum residue levels for acetamiprid, aclonifen, deltamethrin, oxathiapiprolin and potassium phosphonates in or on certain products
Draft Annex II
What is changing and why?
The European Union (EU) is increasing the maximum residue level (MRL) for acetamiprid on honey and other apiculture products from 0.3 to 1 mg/kg, after a modification of the existing MRLs was requested. The European Food Safety Authority has concluded that the new MRL proposed for honey and other apiculture products is safe for consumers.
This follows MRL increases for acetamiprid in June 2025 on a range of products including honey. In January 2025, the MRLs for acetamiprid were reduced on many other products including asparagus, bananas, chards, currants, escaroles, lettuces, and spinach (see Table 1 for details).
Actions
Suppliers to the EU market of asparagus, bananas, chards, currants, escaroles, lettuces, and spinach should review their current use of acetamiprid and seek alternative solutions. Suppliers of other affected products should review their use of acetamiprid and assess whether any changes will be needed to existing good agricultural practices (GAP).
A new review of this substance will be carried out in February 2027. Suppliers should continue to monitor levels of acetamiprid and the GAP for its use in apples, pears, quinces, apricots, sweet peppers, cucumbers, and courgettes, so that data can be submitted to the European Commission before 24 September 2026.
Timeline
The new MRL for honey and other apiculture products is expected to apply from February 2026.
The MRLs adopted under Regulation 2025/158 apply from 19 August 2025. Suppliers that exported products before 19 August 2025 must ensure that they comply with the new MRLs when they remain on the market.
The MRLs adopted under Regulation 2025/1212 apply from 20 August 2025.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.