Maximum residue levels for chlorfenapyr
- Food safety
- Pesticide MRLs
Summary
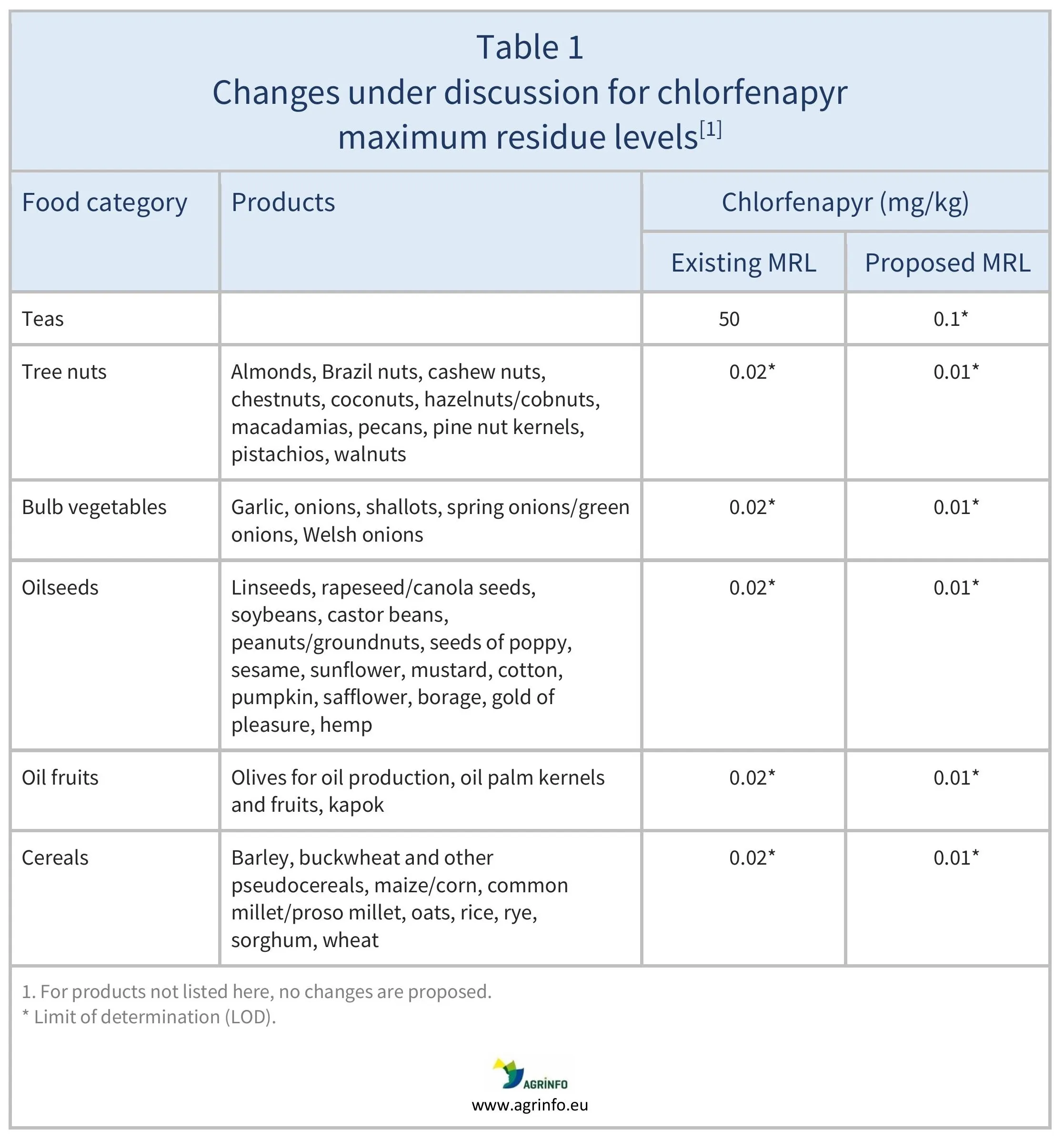
The European Union (EU) is discussing reducing the maximum residue level (MRL) for chlorfenapyr on tea from 50 mg/kg to the limit of determination (LOD) of 0.01 mg/kg. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.) Minor reductions in current LODs are also proposed for certain other products.
EU discusses reduction of chlorfenapyr MRL for tea to 0.01 mg/kg
Draft Commission Regulation amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for azocyclotin, chlorfenapyr, cyhexatin, diazinon, dicofol, endosulfan, fenarimol, fenpropathrin and profenofos in or on certain products
Draft Annex
Update
The European Union (EU) is discussing reducing the maximum residue level (MRL) for chlorfenapyr on tea from 50 mg/kg to the limit of determination (LOD) of 0.01 mg/kg. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.) Minor reductions in current LODs are also proposed for certain other products.
Impacted Products
Almonds, Brazil nuts, cashew nuts, chestnuts, coconuts, hazelnuts/cobnuts, macadamias, pecans, pine nut kernels, pistachios, walnuts, garlic, onions, shallots, spring onions/green onions, Welsh onions, linseeds, peanuts/groundnuts, poppy seeds, sesame seeds, sunflower seeds, rapeseed/canola seeds, soyabeans, mustard seeds, cotton seeds, pumpkin seeds, safflower seeds, borage seeds, gold of pleasure seeds, hemp seeds, castor beans, olives for oil production, oil palm kernels, oil palm fruits, kapok, barley, buckwheat and other pseudocereals, maize/corn, common millet/proso millet, oats, rice, rye, sorghum, wheat
What is changing?
The EU is discussing the reduction of MRLs for chlorfenapyr as summarised in Table 1.
Why?
The use of chlorfenapyr has never been approved for use within the EU, and the MRLs for imported produce that have been in place since the adoption of Regulation 396/2005 have never been reviewed. Following a series of evaluations and a stakeholder consultation (see EFSA invites submission of data to support review of certain MRLs), the European Food Safety Authority has concluded that data gaps regarding the MRL for tea do not allow a complete exposure assessment (EFSA 2023).
Timeline
This Regulation is still under discussion. It is expected to be adopted in 2026, with new MRLs applying from late 2026 or early 2027.
Recommended Actions
Suppliers of tea to the EU market should review their current use of chlorfenapyr and start to seek alternative (chemical or non-chemical) solutions in anticipation of the MRL reduction.
Background
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
For further information on the EU’s process and principles for setting MRLs, see Regulation of pesticide residues in the EU – Questions and Answers.
Resources
EFSA (2024) Outcome of the stakeholder consultation on the reasoned opinions for azocyclotin, bifenthrin, chlorfenapyr, cyhexatin, diazinon, dicofol, endosulfan, fenarimol, fenpropathrin and profenofos. EFSA supporting publication: EN-9002.
Sources
Draft Commission Regulation as regards maximum residue levels for azocyclotin, chlorfenapyr, cyhexatin, diazinon, dicofol, endosulfan, fenarimol, fenpropathrin and profenofos in or on certain products
Draft Annex
Tables & Figures
Source: based on PLAN/2025/1425 Rev0
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU discusses reduction of chlorfenapyr MRL for tea to 0.01 mg/kg
Draft Commission Regulation as regards maximum residue levels for azocyclotin, chlorfenapyr, cyhexatin, diazinon, dicofol, endosulfan, fenarimol, fenpropathrin and profenofos in or on certain products
Draft Annex
What is changing and why?
The European Union (EU) is discussing the reduction of chlorfenapyr maximum residue levels (MRLs) on several products. The most significant likely impacts are on tea, with a reduction from 50 to 0.01 mg/kg (see Table 1). This is because the European Food Safety Authority was unable to complete a risk assessment of the tea MRL due to a lack of relevant data. Chlorfenapyr has never been approved for use within the EU.
Actions
Suppliers of tea to the EU market should review their current use of chlorfenapyr and start to identify alternative pesticides.
Timeline
This Regulation is still under discussion. It is expected to be adopted in 2026, with new MRLs applying from late 2026 or early 2027.
Tables & Figures

Source: based on PLAN/2025/1425 Rev0
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
