Maximum residue levels for chlormequat
- Food safety
- Pesticide MRLs
Summary
The European Commission has informed the World Trade Organization Sanitary and Phytosanitary Measures (WTO SPS) Committee (G/SPS/N/EU/899) that it intends to reduce chlormequat maximum residue levels (MRLs) on animal products and cultivated fungi.
EU discusses reduction of chlormequat MRLs on animal products
Draft Commission Regulation amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for 1,4-dimethylnaphthalene, chlormequat, metribuzin, metribuzin-desamino-diketo (metribuzin-DADK), terbuthylazine and triclopyr in or on certain products.
Draft Annex
Update
The European Commission has informed the World Trade Organization Sanitary and Phytosanitary Measures (WTO SPS) Committee (G/SPS/N/EU/899) that it intends to reduce chlormequat maximum residue levels (MRLs) on animal products and cultivated fungi.
Impacted Products
Fungi, mosses, lichens, swine (all), cattle (all), sheep (liver), sheep (fat), sheep (edible offals), goat (all), horse (all), poultry (all), other farmed terrestrial animals (all), milk (all), bird eggs (domestic fowl)
What is changing?
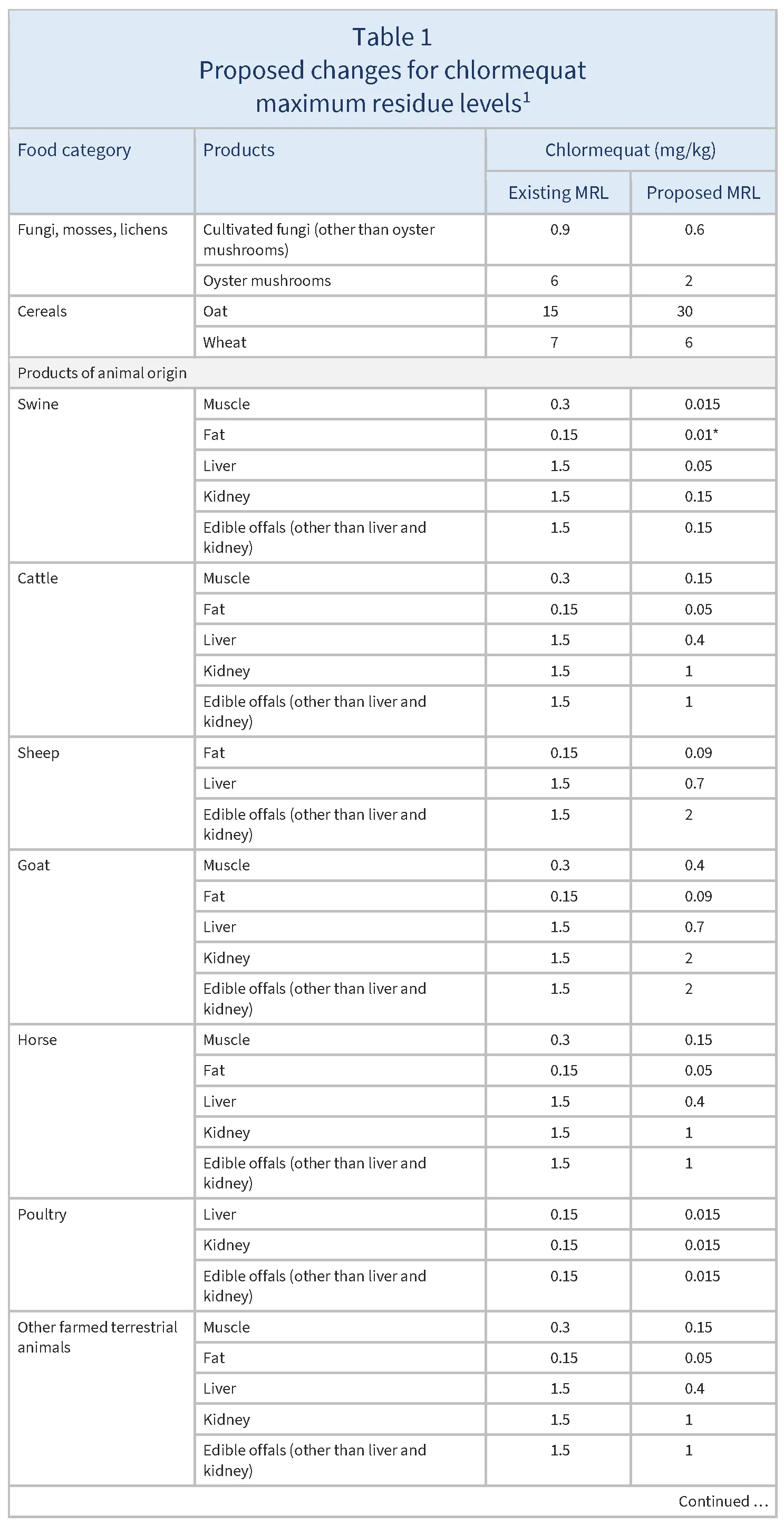
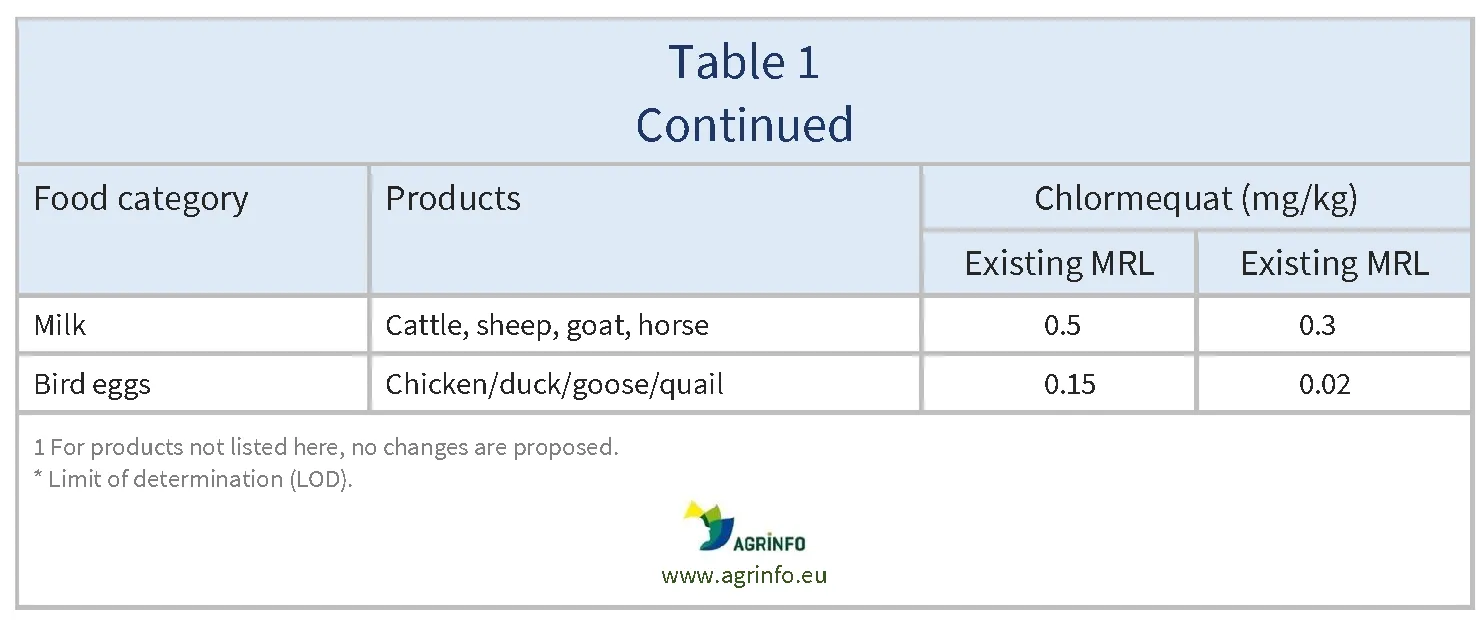
The European Union (EU) proposes to reduce the MRLs for chlormequat on certain products as summarised in Table 1.
Why?
Food operators have submitted recent monitoring data showing that chlormequat residues still occur in oyster mushrooms and cultivated fungi at levels higher than the limit of determination (LOD, the lowest level that can be detected using the most modern and reliable analytical methods). As the majority of residues in the new monitoring data are lower than the current MRL, the MRL can be lowered somewhat, although not all the way to the LOD. For example, for oyster mushrooms, new data show that the majority of residues are under 2 mg/kg. This means that the MRL can be lowered from the previous level (6 mg/kg) to 2 mg/kg, but not to the LOD of 0.01 mg/kg.
Chlormequat may be used on oilseeds or cereals used as feed, with potential carry-over of chlormequat residues in products of animal origin. Following an evaluation, the European Food Safety Authority (EFSA 2024) has concluded that this carry-over could be accommodated by lowering MRLs for most animal products except for sheep kidney, and for poultry muscle and fat.
Timeline
The Regulation is expected to be published in July 2026. It is expected that new MRLs will apply from late 2026 or early 2027.
Recommended Actions
The WTO consultation on this draft Regulation closed on 1 February 2026.
Background
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
For further information on the EU’s process and principles for setting MRL, see Regulation of pesticide residues in the EU – Questions and Answers.
Resources
EFSA (2024) Assessment of fall-back MRLs for revoked CXLs previously implemented in the EU legislation and review of the JMPR evaluation of the toxicological data related to pyrasulfotole, pyraziflumid, spiropidion and tetraniliprole. EFSA Journal, 22(4): art. e8693.
Sources
Draft Commission Regulation as regards maximum residue levels for 1,4-dimethylnaphthalene, chlormequat, metribuzin, metribuzin-desamino-diketo (metribuzin-DADK), terbuthylazine and triclopyr in or on certain products.
Draft Annex
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU discusses reduction of chlormequat MRLs on animal products
Draft Commission Regulation as regards maximum residue levels for 1,4-dimethylnaphthalene, chlormequat, metribuzin, metribuzin-desamino-diketo (metribuzin-DADK), terbuthylazine and triclopyr in or on certain products.
Draft Annex
What is changing and why?
The European Commission has informed the World Trade Organization Sanitary and Phytosanitary Measures (WTO SPS) Committee that it intends to reduce chlormequat maximum residue levels (MRLs) on animal products and cultivated fungi (see Table 1).
Food operators have submitted recent monitoring data showing that chlormequat residues still occur in oyster mushrooms and cultivated fungi at levels higher than the limit of determination (LOD, the lowest level that can be detected using the most modern and reliable analytical methods). As the majority of residues in the new monitoring data are lower than the current MRL, the MRL can be lowered somewhat, although not all the way to the LOD.
The MRLs are lowered for most animal products except for sheep kidney, and for poultry muscle and fat. This is because chlormequat residues can be carried over to products of animal origin when it is used on oilseeds or cereals used as feed.
Actions
The WTO consultation on this draft Regulation closed on 1 February 2026.
Timeline
The Regulation is expected to be published in July 2026. It is expected that new MRLs will apply from late 2026 or early 2027.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.