Maximum residue levels for dimoxystrobin
- Food safety
- Pesticide MRLs
Summary
The European Union (EU) has lowered the maximum residue levels (MRLs) for dimoxystrobin to the limit of determination (LOD) on all products. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.) Revised MRLs will have impacts particularly on exporters of sunflower seeds, rye, and wheat.
EU amends MRLs for dimoxystrobin
Commission Regulation (EU) 2026/215 of 29 January 2026 amending Annexes II and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for dimoxystrobin, ethephon and propamocarb in or on certain products
Update
The European Union (EU) has lowered the maximum residue levels (MRLs) for dimoxystrobin to the limit of determination (LOD) on all products. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.) Revised MRLs will have impacts particularly on exporters of sunflower seeds, rye, and wheat.
Impacted Products
Sunflower seeds, rapeseeds/canola seeds, mustard seeds, rye, wheat, animal products (muscle, fat, liver, kidney, edible offals) from swine, cattle, sheep, goats, horses, poultry, other farmed animals, birds’ eggs (chicken, duck, geese, quail), amphibians and reptiles, terrestrial invertebrate animals, wild terrestrial vertebrate animals
What is changing?
The EU has amended the MRLs for dimoxystrobin as summarised in Table 1.
In addition, the EU has lowered the LOD for dimoxystrobin on oilseeds and oil fruits from 0.05 to 0.01 mg/kg, and on animal products from 0.03 to 0.01 mg/kg.
Why?
Because the EU’s approval of dimoxystrobin has not been renewed (Regulation 2023/1436), the MRLs have been lowered to the LOD on all products. See Dimoxystrobin: non-renewal of EU approval.
The EU Reference Laboratories for pesticide residues were consulted to assess the need to adapt certain LODs. For dimoxystrobin, the laboratories have proposed product-specific LODs that are analytically achievable, ensuring compliance with updated safety and monitoring standards.
Timeline
The new MRLs apply from 19 August 2026.
Recommended Actions
Suppliers of sunflower seeds, rye, and wheat in particular should evaluate their current use of dimoxystrobin and explore possible alternative solutions in anticipation of these MRL changes.
Background
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
Commission Implementing Regulation (EU) 2023/1436 concerning the non-renewal of the approval of the active substance dimoxystrobin
Sources
Commission Regulation (EU) 2026/215 as regards maximum residue levels for dimoxystrobin, ethephon and propamocarb in or on certain products
Tables & Figures
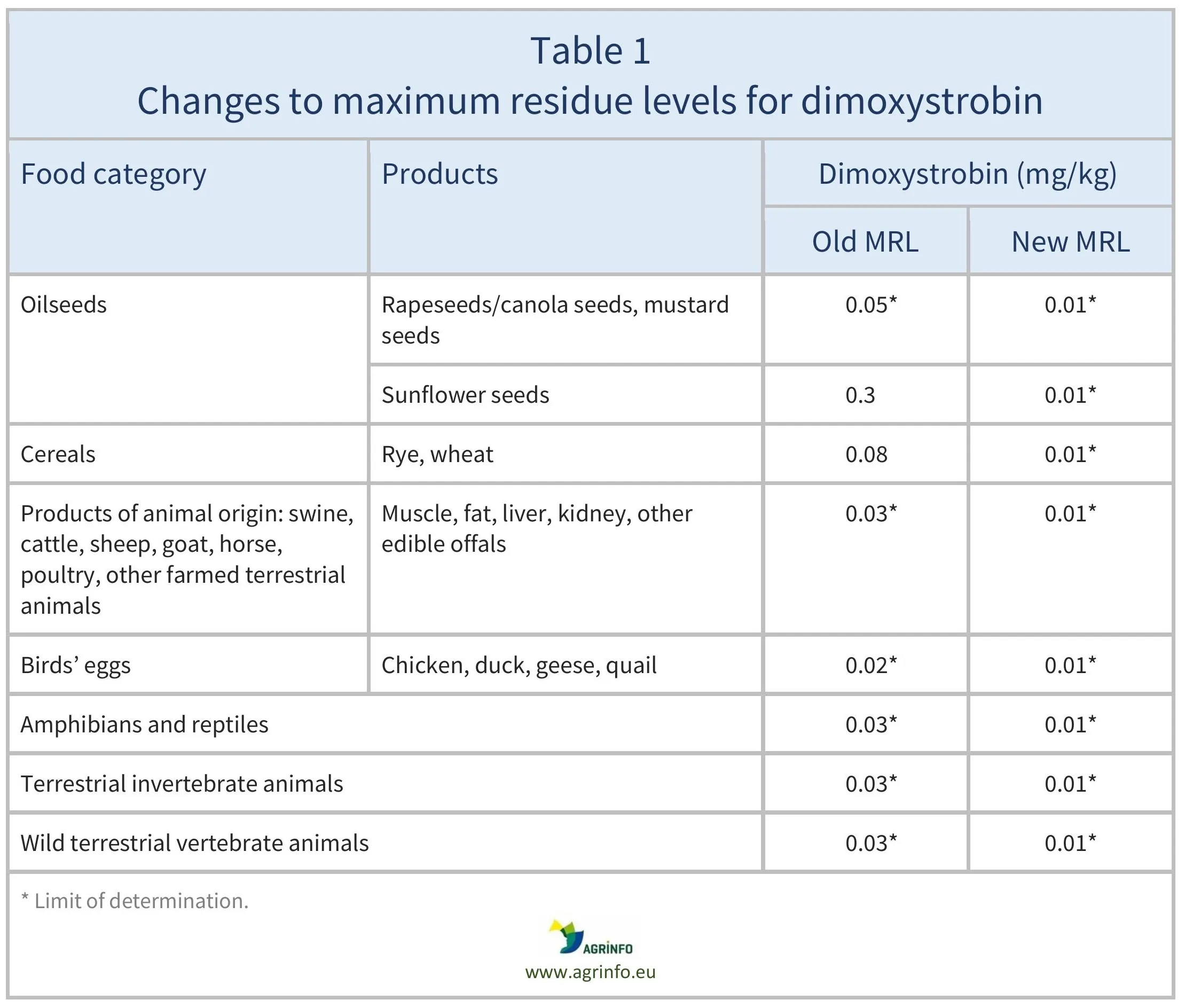
Source: based on Regulation 2026/215
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU amends MRLs for dimoxystrobin
Commission Regulation (EU) 2026/215 as regards maximum residue levels for dimoxystrobin, ethephon and propamocarb in or on certain products
What is changing and why?
Because the European Union (EU) has not renewed its approval of dimoxystrobin, the maximum residue levels (MRLs) have been lowered to the limit of determination (LOD) on all products. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.) See Dimoxystrobin: non-renewal of EU approval.
The changes are set out in Table 1.
In addition, the EU has lowered the LOD for dimoxystrobin on oilseeds and oil fruits from 0.05 to 0.01 mg/kg, and on animal products from 0.03 to 0.01 mg/kg.
Actions
Suppliers of sunflower seeds, rye, and wheat in particular should evaluate their current use of dimoxystrobin and explore possible alternative solutions in anticipation of these MRL changes.
Timeline
The new MRLs apply from 19 August 2026.
Tables & Figures

Source: based on Regulation 2026/215
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
