Maximum residue levels for fosetyl-Al/phosphonic acid
- Food safety
- Pesticide MRLs
Summary
The European Commission has changed the residue definition for fosetyl-Al, potassium phosphonates, and disodium phosphonates to “phosphonic acid and its salts”, and has updated the existing maximum residue levels (MRLs) for these substances in certain commodities. This may have a particular impact on suppliers of sweetcorn, herbal infusions from flowers, and certain spices. In these cases the MRL is reduced to the limit of determination (LOD, the lowest level that can be detected using the most modern and reliable analytical methods).
EU revises fosetyl-Al MRLs, with impacts on sweetcorn and certain herbal infusions/spices
Commission Regulation (EU) 2024/2619 of 8 October 2024 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for fosetyl, potassium phosphonates and disodium phosphonate in or on certain products
Update
The European Commission has changed the residue definition for fosetyl-Al, potassium phosphonates, and disodium phosphonates to “phosphonic acid and its salts”, and has updated the existing maximum residue levels (MRLs) for these substances in certain commodities. This may have a particular impact on suppliers of sweetcorn, herbal infusions from flowers, and certain spices. In these cases the MRL is reduced to the limit of determination (LOD, the lowest level that can be detected using the most modern and reliable analytical methods).
Impacted Products
Citrus fruits, tree nuts, pome fruits, stone fruits, berries and small fruits, miscellaneous fruits, root and tuber vegetables, bulb vegetables, fruiting vegetables, Brassica vegetables, leaf vegetables, herbs and edible flowers, legume vegetables, stem vegetables, fungi, mosses and lichens, algae and prokaryotes, pulses, oilseeds and oil fruits, cereals, teas, coffee, herbal infusions, cocoa and carobs, hops, spices, sugar plants, muscle, fat, liver, kidney and edible offals from swine, cattle, sheep, goat, horses, poultry and other farmed land animals, milk, eggs, honey
What is changing?
The European Commission has changed the residue definition for three active substances – fosetyl-Al, potassium phosphonates, and disodium phosphonate – from “fosetyl-Al (sum of fosetyl, phosphonic acid and their salts, expressed as fosetyl)” to “phosphonic acid and its salts, expressed as phosphonic acid”.
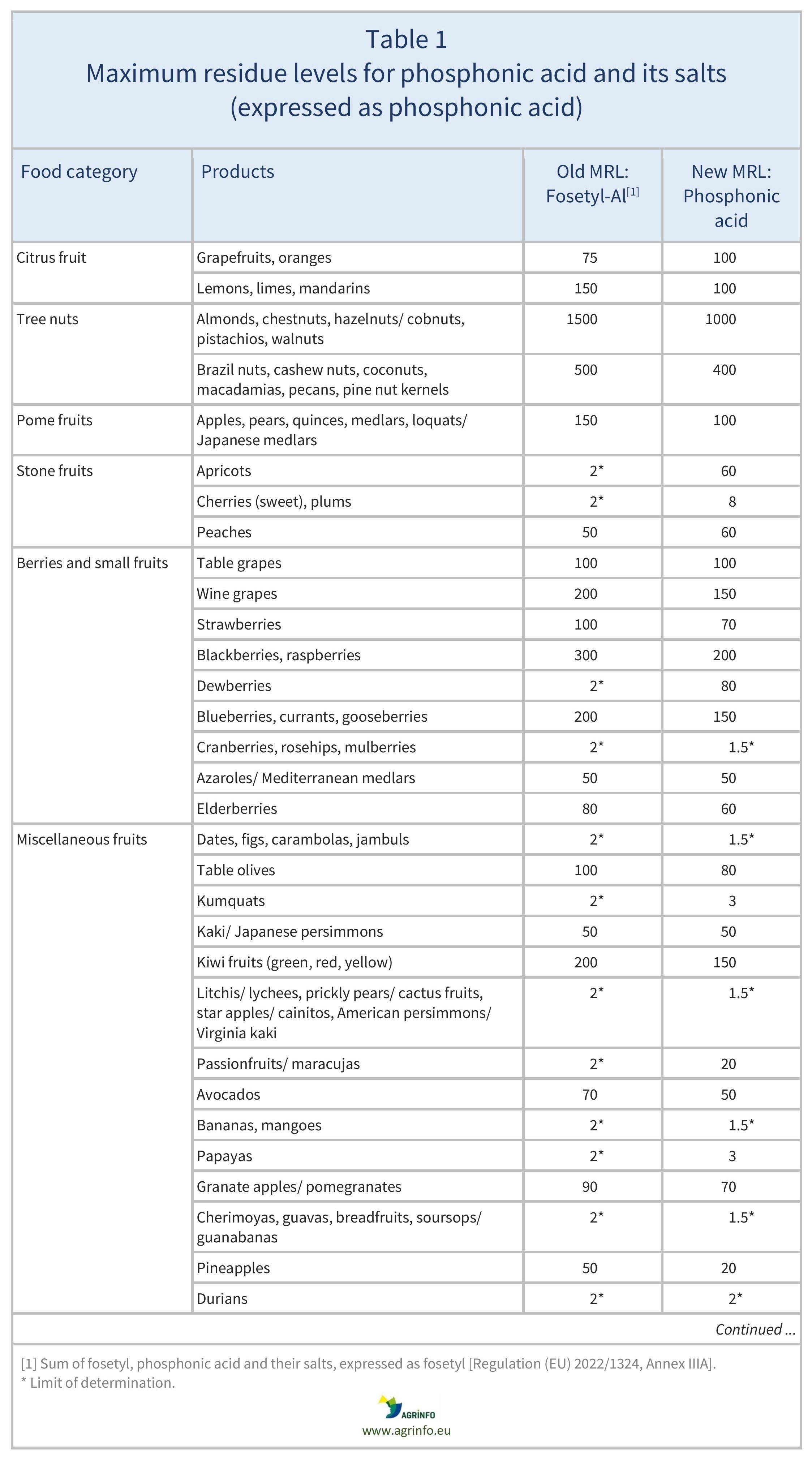
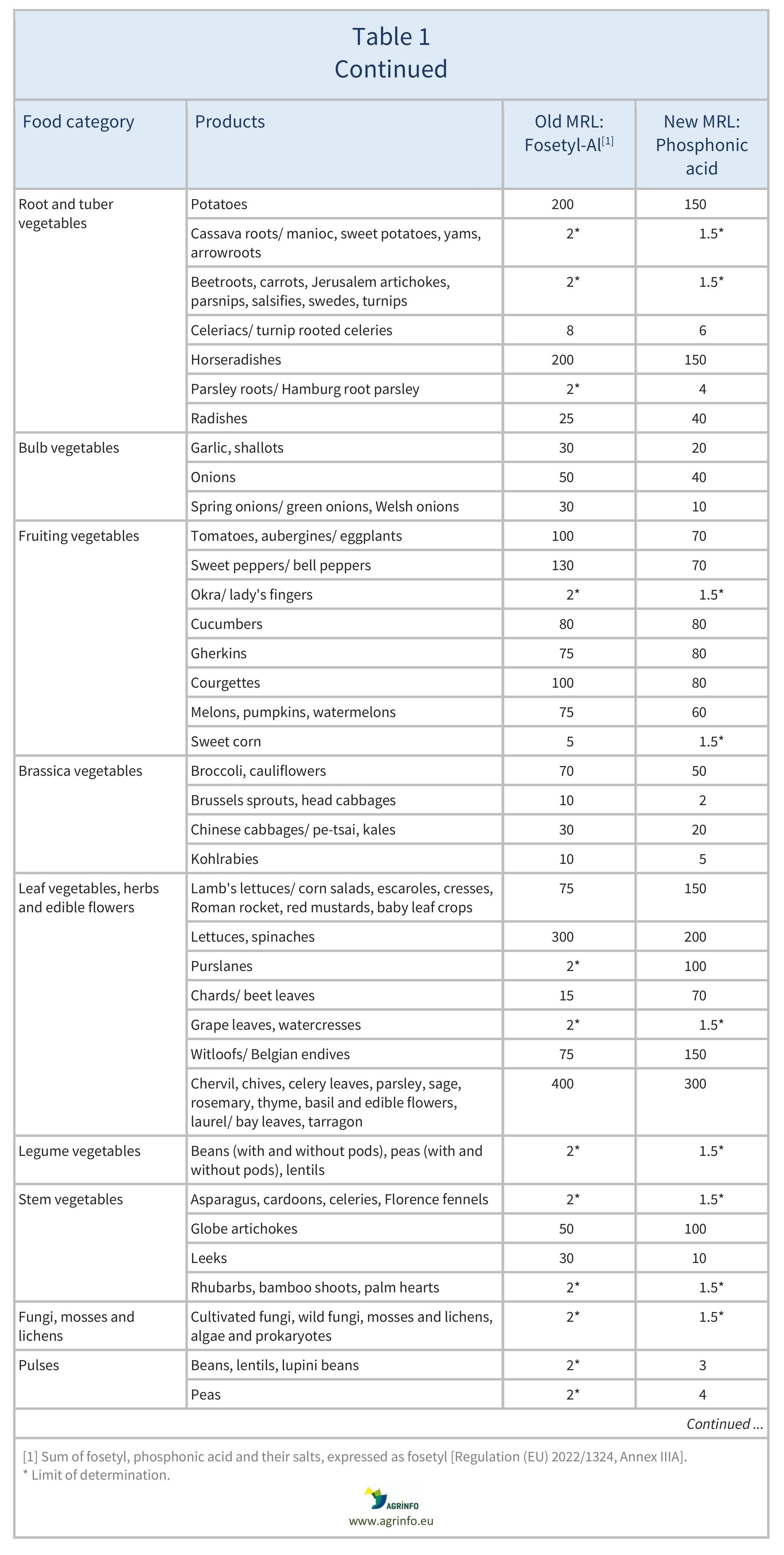
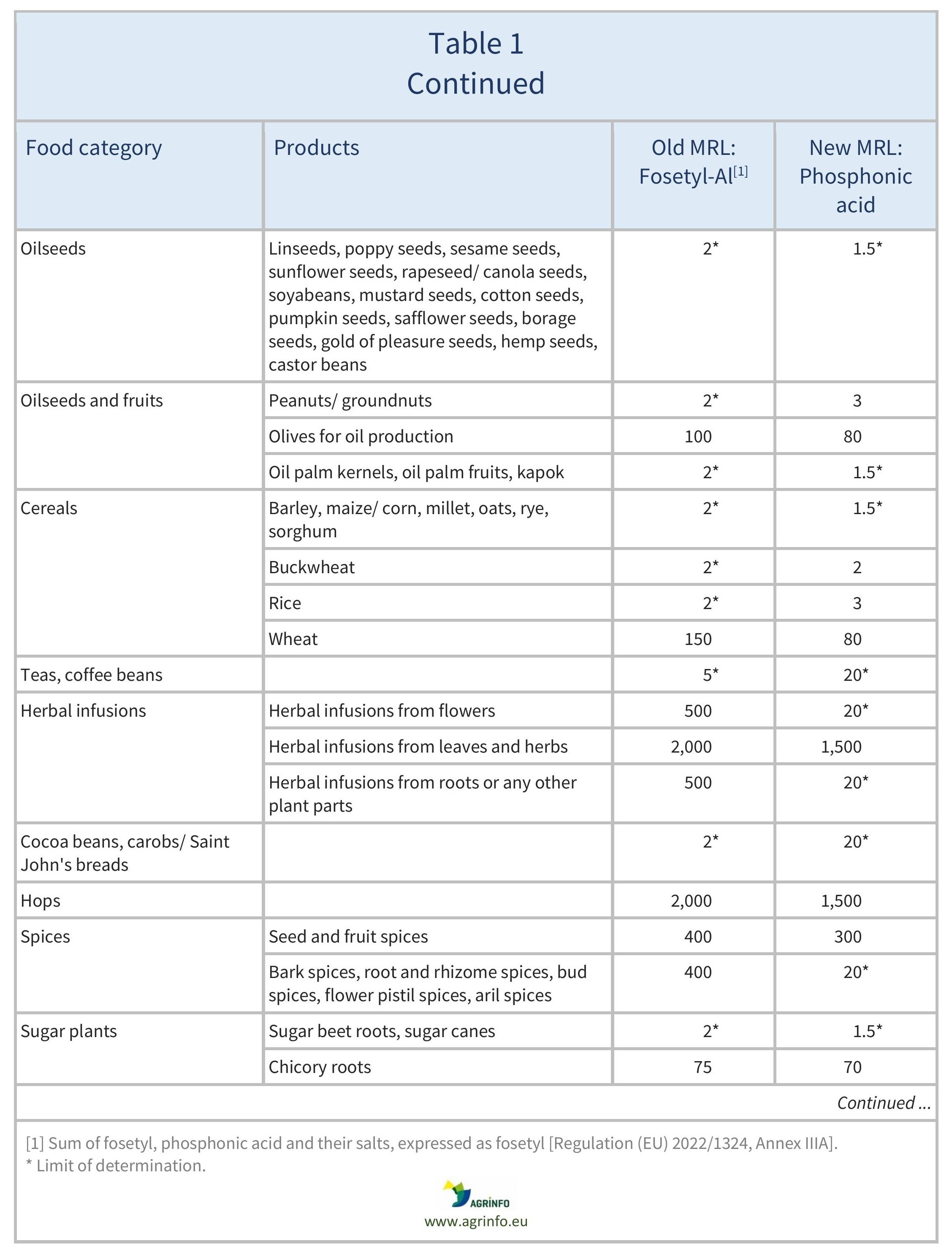
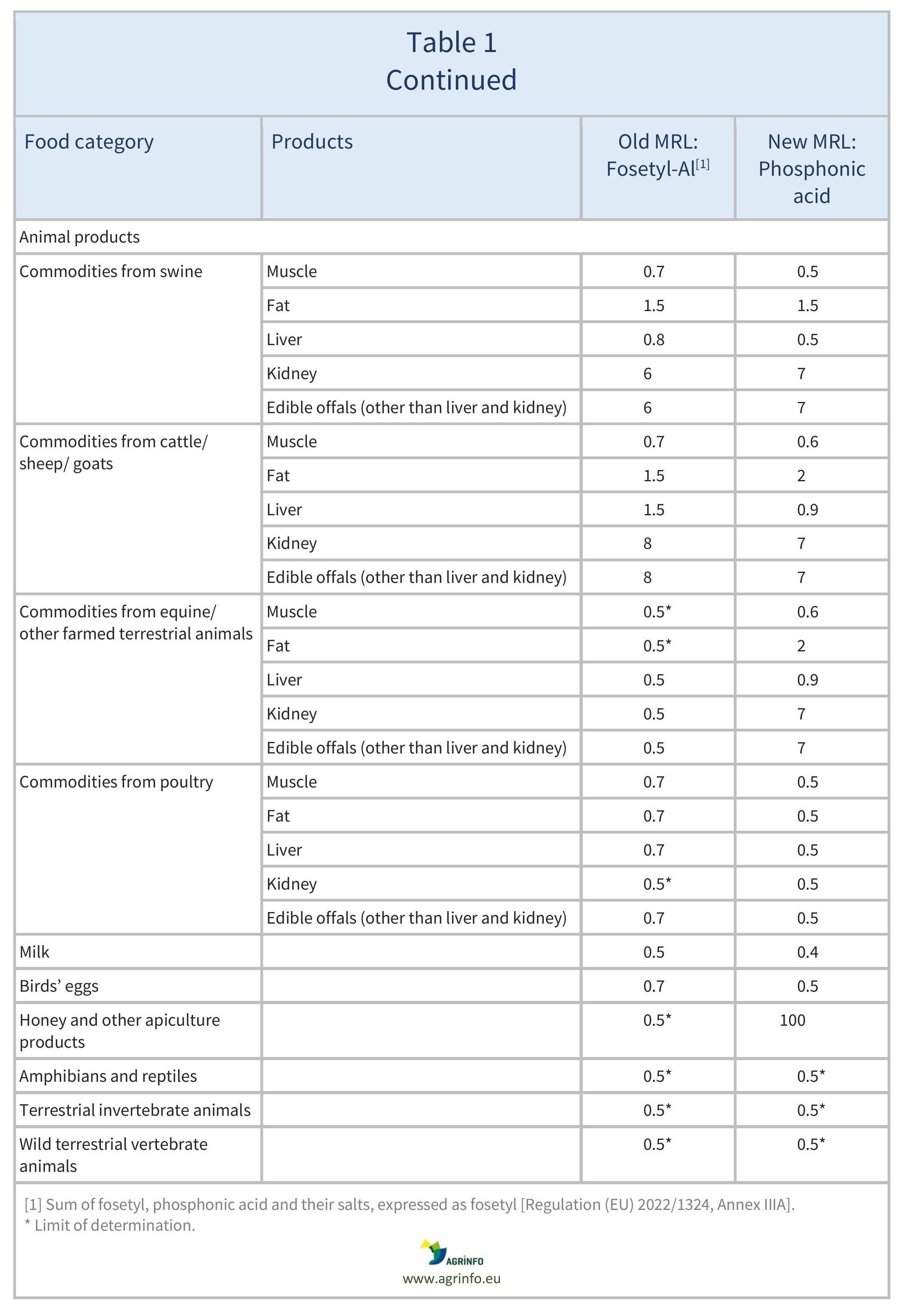
The MRLs for fosetyl-Al have been reviewed based on this new definition. The MRLs for sweetcorn, herbal infusions from flowers, and certain spices (bark spices, root and rhizome spices, bud spices, flower pistil spices) have been reduced to the LOD. A comparison of previous fosetyl-Al and the new phosphonic acid MRLs can be found in Table 1.
Why?
The residue definition for fosetyl-Al, potassium phosphonates, and disodium phosphonates to “phosphonic acid and its salts” has been changed on the recommendation of EFSA (2021).
These active substances all degrade to phosphonic acid. Their residues must be jointly assessed when setting MRLs.
Timeline
The new MRLs apply from 29 April 2025.
Recommended Actions
These substances are not only used in plant protection products, but also as ingredients in fertilisers, plant strengtheners, manure, and soil amendments. Suppliers of all products should assess their compliance with the new MRLs.
Suppliers of sweetcorn, herbal infusions from flowers, and spices (bark spices, root and rhizome spices, bud spices, flower pistil spices) should review their current uses of fosetyl-Al, potassium phosphonates, and disodium phosphonate as the MRL is reduced to the LOD. Suppliers of these products should look for possible alternative solutions.
Background
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
EFSA (2021) Reasoned opinion on the joint review of maximum residue levels (MRLs) for fosetyl, disodium phosphonate and potassium phosphonates according to Articles 12 and 43 of Regulation (EC) No 396/2005. EFSA Journal 19(8): 6782.
Sources
Regulation (EU) 2024/2619 as regards maximum residue levels for fosetyl, potassium phosphonates and disodium phosphonate in or on certain products
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU revises fosetyl-Al MRLs, with impacts on sweetcorn and certain herbal infusions/spices
Regulation (EU) 2024/2619 as regards maximum residue levels for fosetyl, potassium phosphonates and disodium phosphonate in or on certain products
What is changing and why?
The European Commission has revised the residue definition of “fosetyl-Al” to “phosphonic acid”. This is because fosetyl-Al, potassium phosphonates, and disodium phosphonate all degrade to phosphonic acid. The Commission has set new maximum residue levels (MRLs) under the new definition. Revised MRLs can be found in Table 1.
Actions
Suppliers of sweetcorn, herbal infusions from flowers, and spices (bark spices, root and rhizome spices, bud spices, flower pistil spices) should review their current use of fosetyl-Al, potassium phosphonates, and disodium phosphonates, and look for possible alternative solutions.
Timeline
The new MRLs apply from 29 April 2025.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.