Maximum residue levels for isopyrazam
- Food safety
- Pesticide MRLs
Summary
The European Commission informed the World Trade Organization Sanitary and Phytosanitary Measures (WTO SPS) Committee in June 2024 that it intends to amend the maximum residue levels (MRLs) for isopyrazam, with possible impacts on fruits and vegetables (G/SPS/N/EU/762).
MRLs will be aligned with Codex MRLs (CXLs) where these are considered by the European Food Safety Authority (EFSA) to present no health risks for the consumer.
Adoption of this proposal was originally foreseen for 2025. However, the Regulation is currently put on hold pending ongoing discussions within the Commission (European Commission 2025a).
Proposal to amend MRLs for isopyrazam, impacting fruit and vegetables on hold
Draft Commission Regulation amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for isopyrazam in or on certain products
Draft Annexx
Update
The European Commission informed the World Trade Organization Sanitary and Phytosanitary Measures (WTO SPS) Committee in June 2024 that it intends to amend the maximum residue levels (MRLs) for isopyrazam, with possible impacts on fruits and vegetables (G/SPS/N/EU/762).
MRLs will be aligned with Codex MRLs (CXLs) where these are considered by the European Food Safety Authority (EFSA) to present no health risks for the consumer.
Adoption of this proposal was originally foreseen for 2025. However, the Regulation is currently put on hold pending ongoing discussions within the Commission (European Commission 2025a).
Impacted Products
Apples, pears, quinces, medlars, loquats/ Japanese medlars, peaches, bananas, beetroots, celeriacs/ turnip rooted celeries, horseradishes, Jerusalem artichokes, parsnips, parsley roots/ Hamburg rooted parsley, radishes, salsifies, swedes/ rutabagas, turnips, carrots, tomatoes, aubergines/ eggplants, cucumbers, gherkins, courgettes, melons, pumpkins, watermelons, chervil, chives, celery leaves, parsley, sage, rosemary, thyme, basil and edible flowers, laurel/ bay leaves, tarragon, linseeds, poppy seeds, mustard seeds, rapeseeds/ canola seeds, oat, rye, wheat, tea, coffee beans, herbal infusions (chamomile, hibiscus, rose, jasmine, lime/ linden, strawberry, rooibos, maté, valerian, ginseng), cocoa beans, carobs/ Saint John’s breads, hops, anise/ aniseed, black caraway/ black cumin, celery, coriander, cumin, dill, fennel, fenugreek, nutmeg, allspice/ pimento, Sichuan pepper, caraway, cardamom, juniper berry, peppercorn, vanilla, tamarind, cinnamon, liquorice, ginger, turmeric/ curcuma, horseradish, cloves, capers, saffron, mace
What is changing?
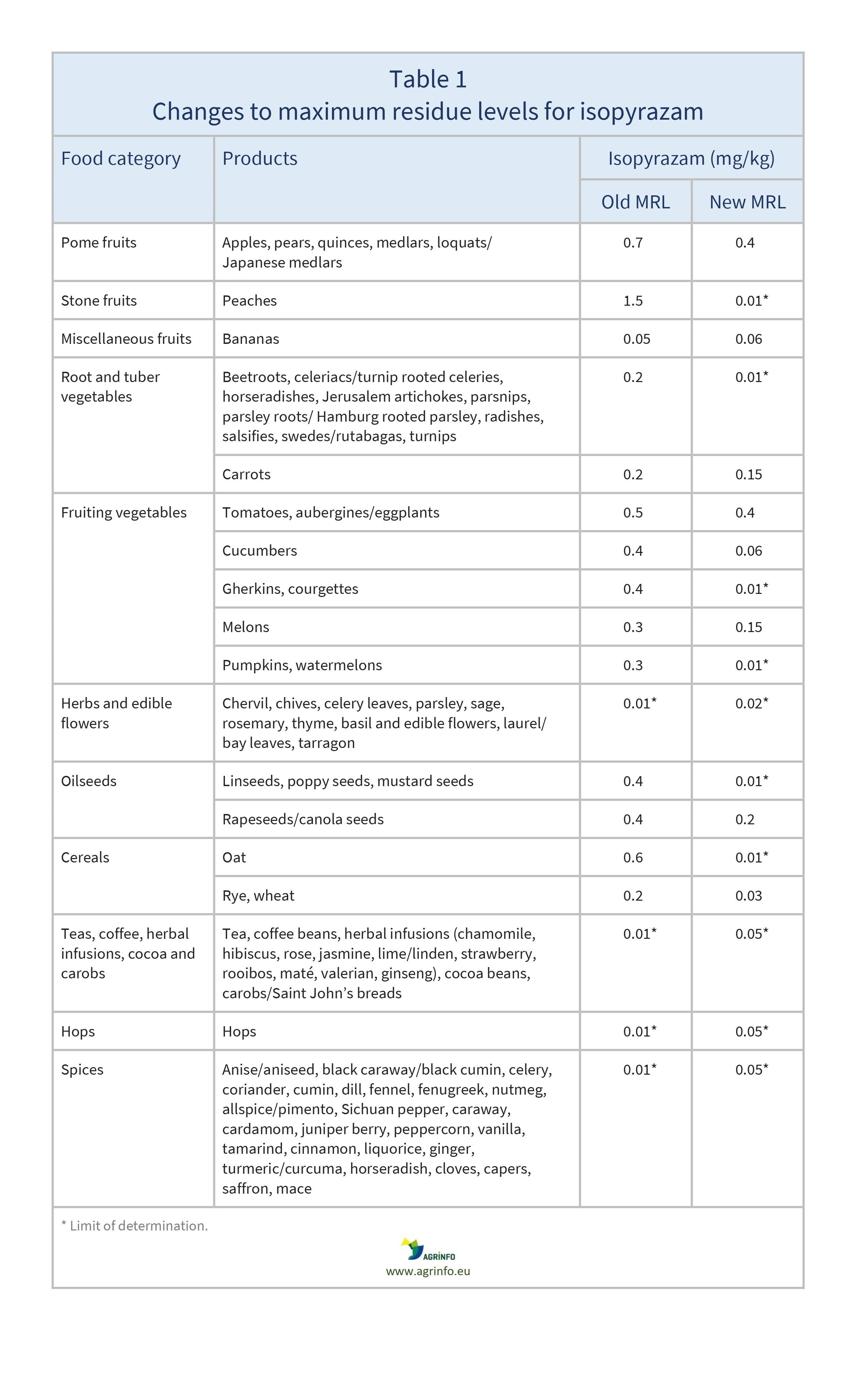
The EU proposes to amend the MRLs for isopyrazam as summarised in Table 1.
Why?
The active substance isopyrazam is no longer approved in the EU because it has been classified as carcinogenic and toxic for reproduction (ECHA 2020). Therefore the EU proposes to reduce the MRLs for this substance to the limit of determination (LOD, the lowest level that can be detected using the most modern and reliable analytical methods). This will apply to all products except those for which MRLs are based on Codex MRLs (CXLs) or import tolerances, which have been reviewed by EFSA (2021) and found to present no health risks for the consumer.
Timeline
Adoption of this proposal was originally foreseen for 2025. However, the Regulation is currently put on hold pending ongoing discussions within the Commission (European Commission 2025a).
Recommended Actions
Suppliers of products affected should review their current use of isopyrazam and look for possible alternative solutions in anticipation of these MRL changes.
Background
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
ECHA (2020) Opinion proposing harmonised classification and labelling at EU level of isopyrazam. European Chemicals Agency, Committee for Risk Assessment.
EFSA (2021) Review of the existing maximum residue levels for isopyrazam according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal, 19(7): 6684.
Sources
Draft Commission Regulation as regards maximum residue levels for isopyrazam in or on certain products
Tables & Figures
Source: based on PLAN/2023/2927
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
Proposal to amend MRLs for isopyrazam, impacting fruit and vegetables on hold
Draft Commission Regulation as regards maximum residue levels for isopyrazam in or on certain products
What is changing and why?
The active substance isopyrazam is no longer approved in the EU because it has been classified as carcinogenic and toxic for reproduction (ECHA 2020). Therefore the EU proposes to reduce the maximum residue levels (MRLs) for this substance to the limit of determination (LOD, the lowest level that can be detected using the most modern and reliable analytical methods). This will apply to all products except those for which MRLs are based on Codex MRLs (CXLs) or import tolerances, which have been reviewed by EFSA (2021) and found to present no health risks for the consumer.
The changes are summarised in Table 1.
Actions
Suppliers of products affected should review their current use of isopyrazam and look for possible alternative solutions in anticipation of these MRL changes.
Timeline
Adoption of this proposal was originally foreseen for 2025. However, the Regulation is currently put on hold pending ongoing discussions within the Commission (European Commission 2025a).
Tables & Figures

Source: based on PLAN/2023/2927
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
