Maximum residue levels for mefentrifluconazole
- Food safety
- Pesticide MRLs
Summary
The European Union (EU) has raised the maximum residue levels (MRLs) for mefentrifluconazole on lettuces and oats, in line with Codex Alimentarius MRLs (CXLs) adopted in 2024.
EU adopts Codex MRLs for mefentrifluconazole on multiple products
Commission Regulation (EU) 2025/1164 of 13 June 2025 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for cyantraniliprole, cyflumetofen, deltamethrin, mefentrifluconazole, mepiquat and oxathiapiprolin in or on certain products
Update
The European Union (EU) has raised the maximum residue levels (MRLs) for mefentrifluconazole on lettuces and oats, in line with Codex Alimentarius MRLs (CXLs) adopted in 2024.
Impacted Products
Lettuces, oats, grapefruits, oranges, lemons, limes, mandarins, almonds, Brazil nuts, cashew nuts, chestnuts, coconuts, hazelnuts/cobnuts, macadamias, pecans, pine nut kernels, walnuts, pistachios, apricots, peaches, cherries (sweet), plums, wine grapes, strawberries, blackberries, dewberries, raspberries (red and yellow), blueberries, currants, gooseberries, rose hips, elderberries, cranberries, mulberries, azaroles, kumquats, avocados, bananas, mangoes, papayas, table olives, kaki/Japanese persimmons, potatoes, cassava roots/manioc, sweet potatoes, yams, arrowroots, beetroots, carrots, celeriacs/turnip rooted celeries, horseradishes, parsnips, parsley roots, radishes, salsifies, swedes, turnips, Jerusalem artichokes, garlic, onions, shallot, spring onions/green onions and Welsh onions, tomatoes, sweet peppers/bell peppers, aubergines/eggplants, okra/lady's fingers, melons, pumpkins, watermelons, sweet corn, cucumbers, gherkins, courgettes, broccoli, cauliflowers, Brussels sprouts, head cabbages, Roman rocket/rucola, baby leaf crops, spinaches, chervil, chives, celery leaves, parsley, sage, rosemary, thyme, basil and edible flowers, laurel/bay leaves, tarragon, beans (with pods), peas (with pods), beans (without pods), peas (without pods), cardoons, celeries, Florence fennels, rhubarbs, globe artichokes, peas, lupins, beans, lentils, linseeds, poppy seeds, mustard seeds, gold of pleasure seeds, sesame seeds, borage seeds, rapeseeds/canola seeds, sunflower seeds, soyabeans, cotton seeds, safflower seeds, barley, common millet/proso millet, rice, rye, wheat, sorghum, coffee beans, ginseng, sugar canes, chicory roots, olives for oil production, hops, swine liver
What is changing?
The EU has raised the MRLs for mefentrifluconazole on lettuces from 0.01 mg/kg (the limit of determination, LOD) to 5 mg/kg; and on oats from 0.6 to 3 mg/kg. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.)
Why?
The new MRLs are in alignment with the new CXLs adopted in November 2024 (CAC 2024). The EU aligns MRLs with CXLs where they are not considered to be a concern for consumer safety following an evaluation by the European Food Safety Authority (EFSA 2024).
Timeline
The new MRLs for lettuces and oats apply from 6 July 2025.
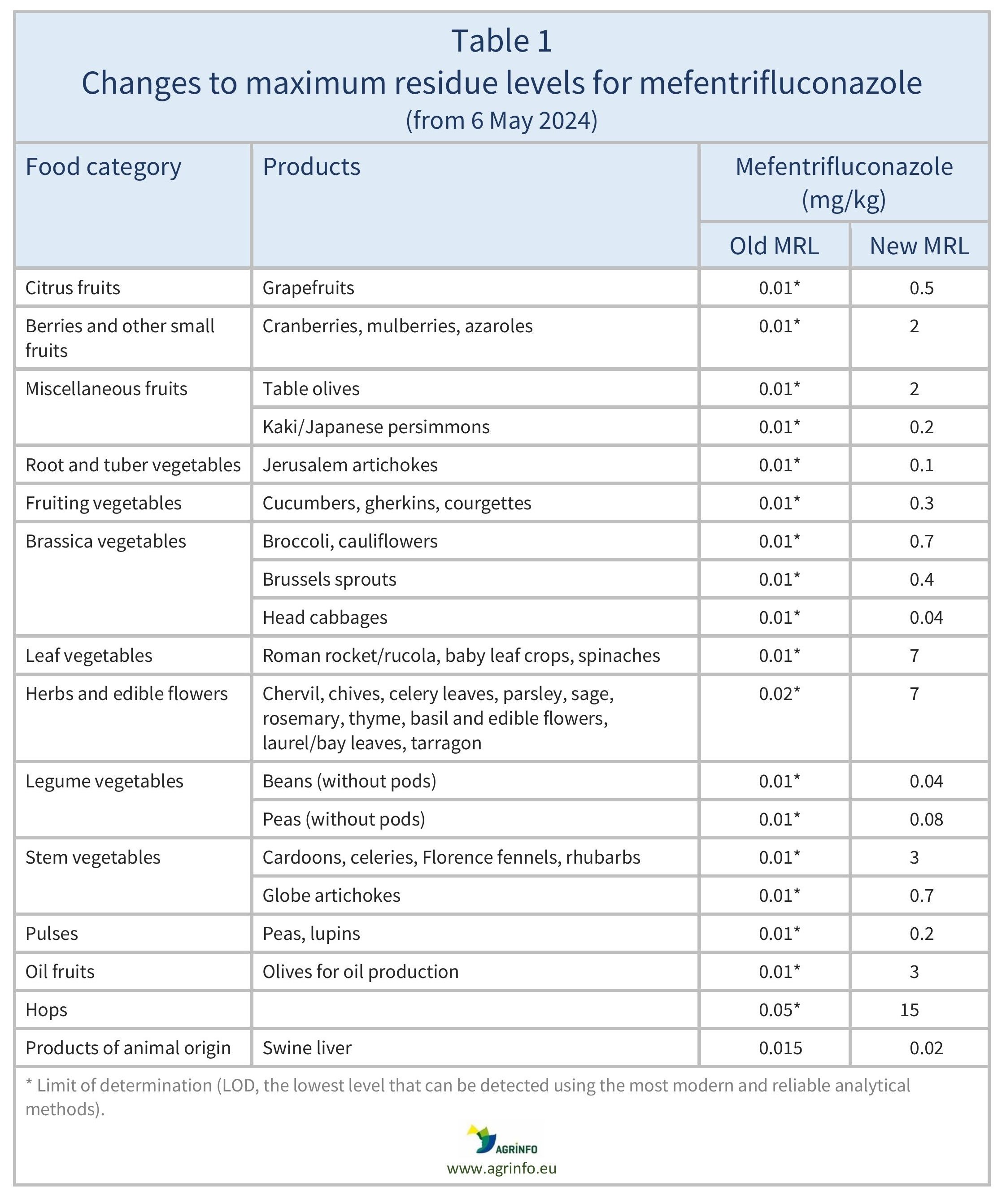
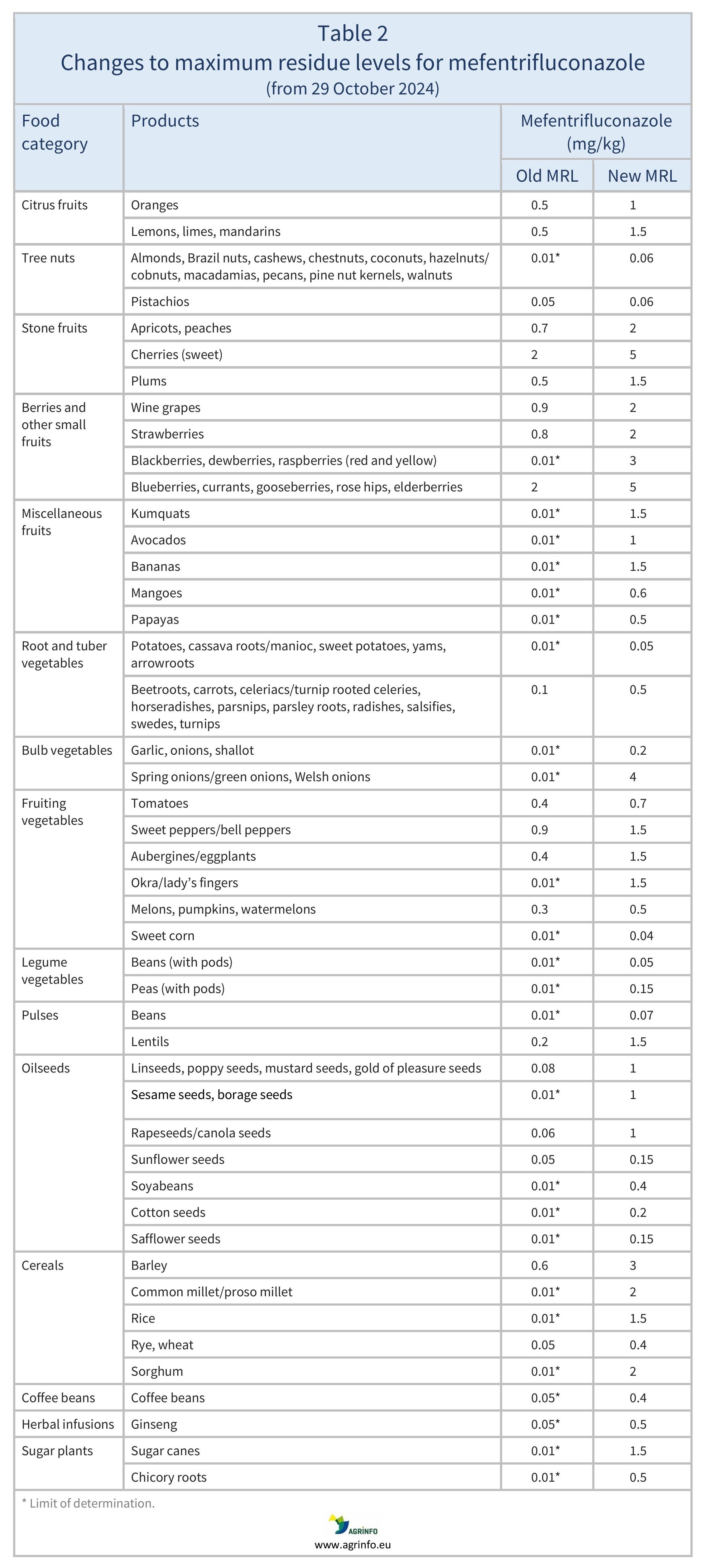
The previous changes to MRLs listed in Table 1 have applied from 6 May 2024, and those listed in Table 2 have applied from 29 October 2024.
Background
In 2024, the EU aligned the MRLs for mefentrifluconazole with the CXLs on various fruits, vegetables, oilseeds/fruits, hops, and swine liver (Regulation 2024/1078) and on multiple products (Regulation 2024/2633). See Tables 1 and 2 for details.
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
CAC (2024) Report of the 55th Session of the Codex Committee on Pesticide Residues, Chengdu, Sichuan Province, P.R. China, 3–8 June 2024. Joint FAO/WHO Food Standards Programme, Codex Alimentarius Commission.
EFSA (2024) Scientific support for preparing an EU position in the 55th Session of the Codex Committee on Pesticide Residues. EFSA Journal, 22(7): e8841.
Regulation (EU) 2024/2633 as regards maximum residue levels for azoxystrobin, famoxadone, flutriafol, mandipropamid and mefentrifluconazole in or on certain products
Regulation (EU) 2024/1078 as regards maximum residue levels for azoxystrobin, flonicamid, isofetamid, mefentrifluconazole, metazachlor, pyrimethanil and quartz sand in or on certain products
Sources
Commission Regulation (EU) 2025/1164 as regards maximum residue levels for cyantraniliprole, cyflumetofen, deltamethrin, mefentrifluconazole, mepiquat and oxathiapiprolin in or on certain products
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU adopts Codex MRLs for mefentrifluconazole on multiple products
Commission Regulation (EU) 2025/1164 as regards maximum residue levels for cyantraniliprole, cyflumetofen, deltamethrin, mefentrifluconazole, mepiquat and oxathiapiprolin in or on certain products
What is changing and why?
The EU has raised the maximum residue levels (MRLs) for mefentrifluconazoleon on lettuces from 0.01 mg/kg (the limit of determination, LOD) to 5 mg/kg; and on oats from 0.6 to 3 mg/kg. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.) These changes bring the EU MRLs into line with Codex MRLs (CXLs) adopted in 2024.
Previous changes to mefentrifluconazole MRLs in 2024 are set out in Tables 1 and 2.
Timeline
The new MRLs for lettuces and oats apply from 6 July 2025.
The previous changes to MRLs listed in Table 1 have applied from 6 May 2024, and those listed in Table 2 have applied from 29 October 2024.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.