Maximum residue levels for trifloxystrobin
- Pesticide MRLs
- Pesticides
Summary
The European Commission is amending the maximum residue levels (MRLs) for trifloxystrobin. This could have a particular impact on passionfruit and Chinese cabbage, as the MRLs for these products are reduced to 0.01 mg/kg. This is the limit of determination (LOD), the lowest level that can be detected using the most modern and reliable analytical methods.
EU amends MRLs for trifloxystrobin with impacts on passionfruit and Chinese cabbage
Commission Regulation (EU) 2024/1342 of 21 May 2024 amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for deltamethrin, metalaxyl, thiabendazole and trifloxystrobin in or on certain products
Corrigendum to Commission Regulation (EU) 2024/1342 of 21 May 2024 amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for deltamethrin, metalaxyl, thiabendazole and trifloxystrobin in or on certain products
Update
The European Commission is amending the maximum residue levels (MRLs) for trifloxystrobin. This could have a particular impact on passionfruit and Chinese cabbage, as the MRLs for these products are reduced to 0.01 mg/kg. This is the limit of determination (LOD), the lowest level that can be detected using the most modern and reliable analytical methods.
Impacted Products
Passionfruits/ maracujas, sweet peppers/ bell peppers, Chinese cabbages/ pe-tsai, kales, chervil, chives, celery leaves, parsley, sage, rosemary, thyme, basil and edible flowers, laurel/ bay leaves, tarragon, beans (with pods), oats, chicory roots, honey
What is changing?
The EU has reduced the MRLs for trifloxystrobin for passionfruit and Chinese cabbage to the LOD of 0.01 mg/kg. Changes to MRLs for other products are summarised in Table 1.
Why?
Following applications to modify several MRLs, EFSA (2022, 2023) did not identify risk to consumers for sweet peppers/ bell peppers, kales, chicory roots, or honey, and suggests MRLs are adopted for these products.
As missing data was not addressed for passionfruit/maracuja or Chinese cabbage/pe-tsai, EFSA suggested replacing those MRLs with the LOD.
To address the remaining data gaps identified by EFSA, the applicant provided new data from residue trials on herbs and edible flowers, beans with pods, and oats. EFSA concluded that the new data sufficiently address the gaps, and that the new MRLs are safe for consumers and should be adopted.
Timeline
The new MRLs will apply from 11 December 2024. Products exported before 11 December 2024 that comply with the old MRLs will not be removed from the EU market after that date, even if they do not comply with the new MRLs.
Recommended Actions
Suppliers of affected products should review their current use of trifloxystrobin and assess whether any changes will be needed to existing good agricultural practices (GAP), or start looking for alternative solutions in anticipation of these MRL changes.
Background
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
EFSA (2022) Modification of existing maximum residue levels in various crops and evaluation of confirmatory data following the Article 12 MRL review for trifloxystrobin. EFSA Journal, 20(1): 7048.
EFSA (2023) Modification of the existing maximum residue level for trifloxystrobin in honey. EFSA Journal, 21(8): 8189.
Sources
Commission Regulation (EU) 2024/1342 as regards maximum residue levels for deltamethrin, metalaxyl, thiabendazole and trifloxystrobin in or on certain products
Tables & Figures
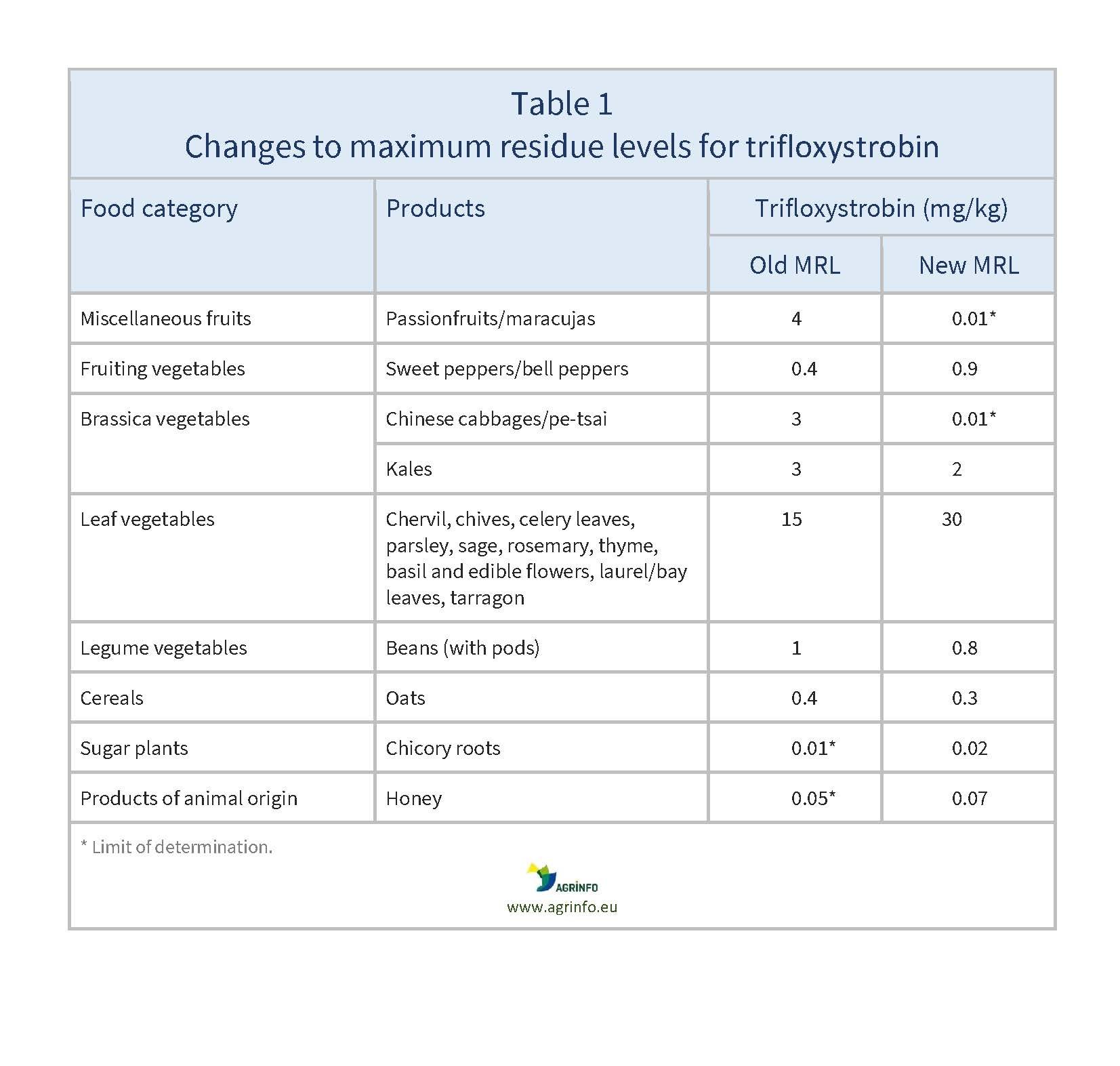
Source: based on Regulation (EU) 2024/1342
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU amends MRLs for trifloxystrobin with impacts on passionfruit and Chinese cabbage
Commission Regulation (EU) 2024/1342 as regards maximum residue levels for deltamethrin, metalaxyl, thiabendazole and trifloxystrobin in or on certain products
What is changing and why?
The EU has decided to reduce the maximum residue levels (MRLs) for trifloxystrobin for passion fruit and Chinese cabbage to 0.01 mg/kg, the limit of determination (LOD, the lowest level that can be detected using the most modern and reliable analytical methods). This change is due to insufficient data.
Changes to MRLs for other products are summarised in Table 1.
Actions
Suppliers of affected products should review their current use of trifloxystrobin and assess whether any changes will be needed to existing good agricultural practices (GAP), or start looking for possible alternative solutions in anticipation of these MRL changes.
Timeline
The new MRLs will apply from 11 December 2024. Products exported before 11 December 2024 that comply with the old MRLs will not be removed from the EU market after that date, even if they do not comply with the new MRLs.
Tables & Figures

Source: based on Regulation (EU) 2024/1342
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
