Maximum residue levels for valifenalate
- Pesticide MRLs
Summary
Commission Regulation (EU) 2022/1346, published in August 2022, reduces the existing MRLs for valifenalate on garlic, lettuces, tomatoes and eggplants (aubergines) from February 2023.
In a further Regulation (2023/129) the MRLs for valifenalate in tomatoes and eggplants have been re-evaluated according to set Codex MRLs, and are now set at 0.4 mg/kg.
EU aligns MRLs for valifenalate on tomatoes and eggplants with Codex standards
Commission Regulation (EU) 2022/1346 of 1 August 2022 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for 1,4-dimethylnaphthalene, 8-hydroxyquinoline, pinoxaden and valifenalate in or on certain products
Commission Regulation (EU) 2023/129 of 18 January 2023 amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for azoxystrobin, prosulfocarb, sedaxane and valifenalate in or on certain products
Update
Commission Regulation (EU) 2022/1346, published in August 2022, reduces the existing MRLs for valifenalate on garlic, lettuces, tomatoes and eggplants (aubergines) from February 2023.
In a further Regulation (2023/129) the MRLs for valifenalate in tomatoes and eggplants have been re-evaluated according to set Codex MRLs, and are now set at 0.4 mg/kg.
Impacted Products
table grapes, wine grapes, garlic, tomatoes, aubergines, eggplants, lettuces
What is changing?
Existing MRLs for valifenalate are amended as shown in Table 1.
The LOD is also increased for herbs and edible flowers from 0.01 to 0.02 mg/kg; and for teas, coffee, infusions, hops and spices from 0.02 to 0.05 mg/kg. For animal products, the LOD is increased from 0.01 to 0.03 mg/kg.
Why?
Following a review of existing MRLs for valifenalate, EFSA (2021a) proposed that the residue definition for products of animal origin should be changed, and the MRLs for certain vegetables should be reduced.
On 14 December 2021, the Codex Alimentarius Commission adopted new Codex maximum residue limits (CXLs) for valifenalate in tomatoes and eggplants. Where international standards exist or will soon be completed, they are taken into consideration in the development or adaptation of food law. EFSA (2021b) assessed the CXLs proposed for valifenalate in tomatoes and eggplants, and concluded they are safe for consumers in the EU. These CXLs are therefore set as EU MRLs.
Timeline
The new MRLs will apply from 22 February 2023. Products lawfully placed on the market in compliance with the old MRLs before 22 February 2023, may remain on the market after that date even if they are not compliant with the new MRLs.
MRLs on tomatoes and eggplant set in Commission Regulation (EU) 2023/129 will apply from 26 February 2023.
Recommended Actions
Suppliers of garlic and lettuces should ensure that alternative solutions to the use of valifenalate on these products are used to ensure conformity with an MRL at the limit of determination (LOD).
Suppliers of tomatoes and eggplants must review existing use of valifenalate to ensure conformity of their products with new reduced valifenalate MRLs by February 2023.
Background
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
EFSA (2021a) Review of the existing maximum residue levels for valifenalate according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal, 19(5): 6591.
EFSA (2021b) Scientific support for preparing an EU position for the 52nd Session of the Codex Committee on Pesticide Residues (CCPR). EFSA Journal, 19(8): 6766.
Sources
Tables & Figures
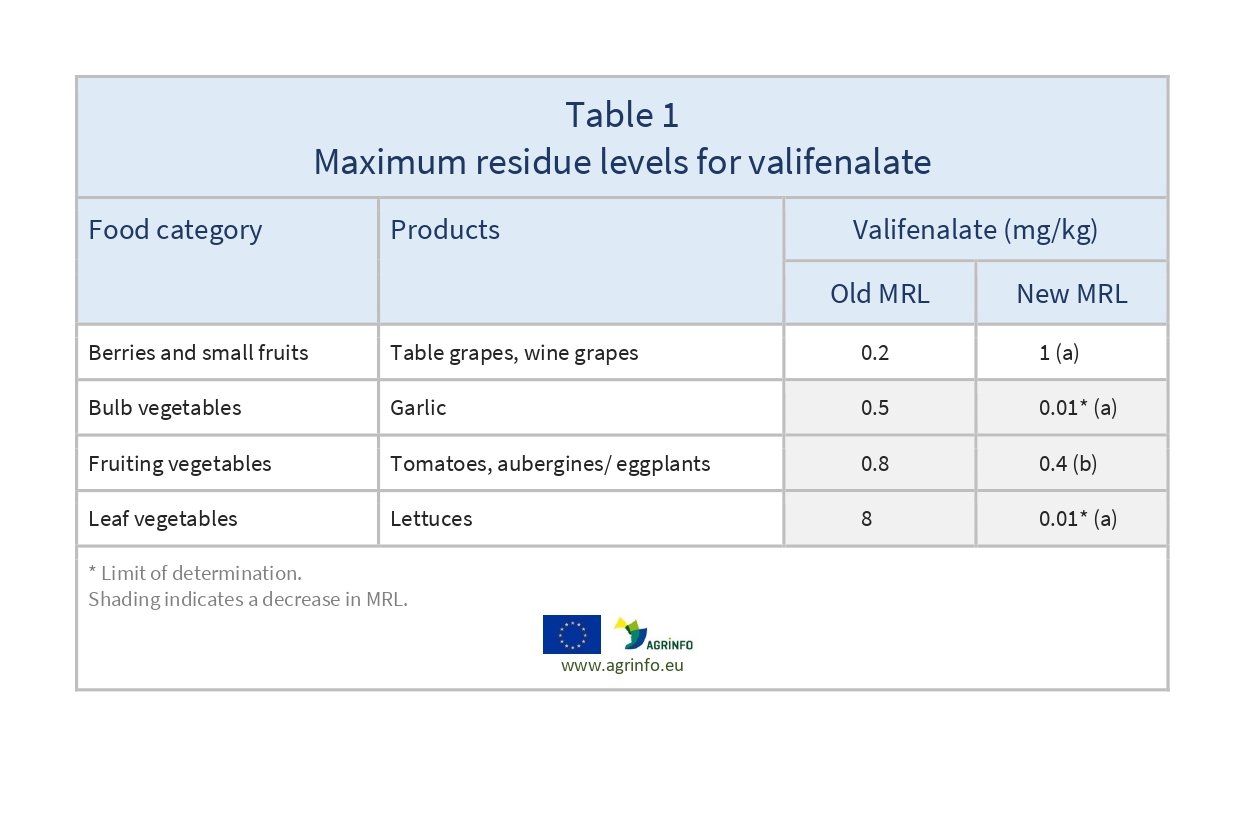
Sources: based on (a) Commission Regulation (EU) 2022/1346; (b) Commission Regulation (EU) 2023/129
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
