Novel food: astaxanthin-rich oleoresin from algae
- Food safety
- Novel/traditional foods
- Food information
- Labelling
Summary
The European Commission has approved a change in the specifications of the novel food astaxanthin-rich oleoresin from Haematococcus pluvialis algae.
EU approves change in specifications for astaxanthin-rich oleoresin from Haematococcus pluvialis algae
Commission Implementing Regulation (EU) 2024/1026 of 8 April 2024 amending Implementing Regulation (EU) 2017/2470 as regards the specifications of the novel food astaxanthin-rich oleoresin from Haematococcus pluvialis algae
Update
The European Commission has approved a change in the specifications of the novel food astaxanthin-rich oleoresin from Haematococcus pluvialis algae.
What is changing?
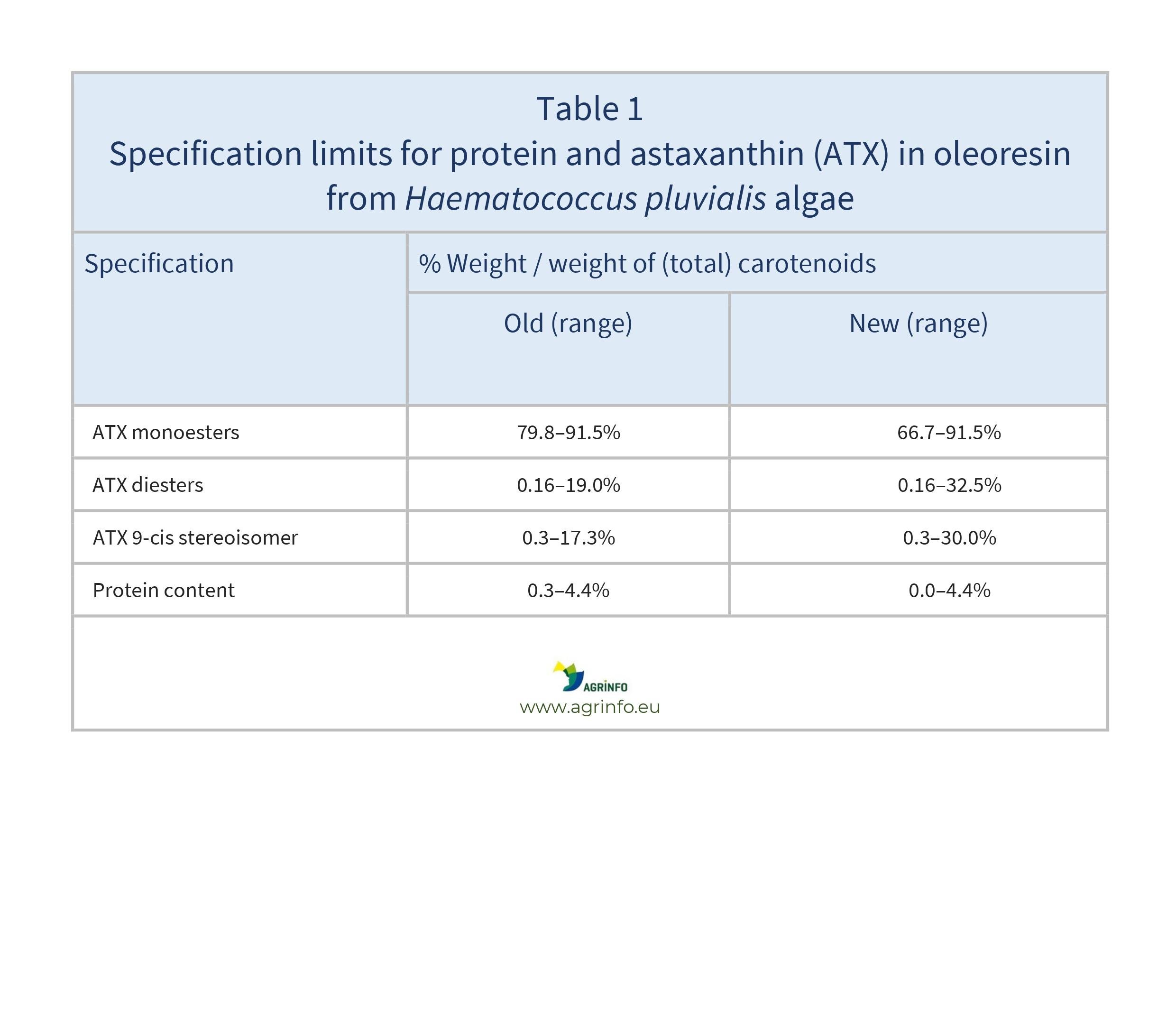
This Regulation authorises lower minimum specification limits for protein and astaxanthin (ATX) monoesters, and increased maximum specification limits for relative amounts of ATX diesters and 9-cis stereoisomers in total ATX (see Table 1).
Why?
EFSA (2023) concluded that the proposed changes in the specifications are safe at the acceptable daily intake (ADI) of ATX from food supplements at 0.2 mg/kg body weight.
Timeline
The new specifications apply from 29 April 2024.
Recommended Actions
Exporters of food supplements containing this substance must ensure that the new specifications set out in Table 1 are respected.
Background
Astaxanthin is a carotenoid produced from H. pluvialis algae, first authorised for use in food supplements for the general population by Regulation (EU) 2017/2470.
Implementing Regulation 2021/1377 limited its conditions of use to adults and adolescents older than 14 years based on an opinion of EFSA (2020).
Implementing Regulation (EU) 2023/1581 authorised its use in food supplements intended for children 3–10 years old and adolescents 10–14 years old, provided that the intake of ATX from food supplements would not exceed an ADI of 0.2 mg/kg body weight per day.
For further information on the novel food authorisation process, see Novel foods explained.
Resources
EFSA (2023) Safety of a change in specifications of the novel food oleoresin from Haematococcus pluvialis containing astaxanthin pursuant to Regulation (EU) 2015/2283. EFSA Journal, 21(11): 5993.
EFSA (2020) Safety of astaxanthin for its use as a novel food in food supplements. EFSA Journal, 18(2): 8338.
Implementing Regulation (EU) 2017/2470 establishing the list of novel foods
Implementing Regulation (EU) 2021/1377 authorising the change of the conditions of use of the novel food astaxanthin-rich oleoresin from Haematococcus pluvialis algae
Implementing Regulation (EU) 2023/1581 as regards the conditions of use of the novel food ‘astaxanthin-rich oleoresin from Haematococcus pluvialis algae’
Sources
Implementing Regulation (EU) 2024/1026 as regards the specifications of the novel food astaxanthin-rich oleoresin from Haematococcus pluvialis algae
Tables & Figures
Source: Implementing Regulation (EU) 2024/1026
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU approves change in specifications for astaxanthin-rich oleoresin from Haematococcus pluvialis algae
Regulation 2024/1026 as regards the specifications of the novel food astaxanthin-rich oleoresin from Haematococcus pluvialis algae
What is changing and why?
The European Commission approved a change in the specifications of the novel food astaxanthin-rich oleoresin from Haematococcus pluvialis algae. Food supplements containing astaxanthin (ATX), a carotenoid, are authorised for adults, for children 3–10 years old, and adolescents 10–14 years old. The European Food Safety Authority (EFSA) concluded that its use is safe as long as the intake of ATX does not exceed the Acceptable Daily Intake (ADI) of 0.2 mg/kg body weight per day. This Regulation authorises lower minimum limits for protein and ATX monoesters, and increased maximum limits for ATX diesters and the 9-cis stereoisomer in total ATX (see Table 1).
Actions
Exporters of food supplements containing this substance must ensure that the new specifications set out in Table 1 are respected.
Timeline
The new specifications apply from 29 April 2024.
Tables & Figures

Source: Implementing Regulation (EU) 2024/1026
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
