Novel food: Calanus oil
- Novel/traditional foods
Summary
The European Commission has updated the specifications (permitted use levels) for the novel food Calanus finmarchicus oil, and the labelling requirements.
European Commission updates specification for novel food Calanus finmarchicus oil
Commission Implementing Regulation (EU) 2025/1513 of 28 July 2025 amending Implementing Regulation (EU) 2017/2470 as regards the conditions of use and the additional labelling requirements of the novel food Calanus finmarchicus oil
Update
The European Commission has updated the specifications (permitted use levels) for the novel food Calanus finmarchicus oil, and the labelling requirements.
What is changing?
Calanus finmarchicus oil was originally authorised under Commission Implementing Decision 2017/2353. The specifications for this novel food (maximum levels and astaxanthin limits) have now been updated (see Table 1).
The labelling requirements have also been updated to reflect the new permitted limits.
Timeline
The new specification applies from 18 August 2025. Food supplements containing Calanus oil on the market or on their way to the EU before 18 August may be sold until their expiry date, provided they comply with Regulation 2017/2470, which was in place prior to these changes.
Background
This Regulation updates the Annex to Regulation 2017/2470 which lists authorised novel foods (see Union list of novel foods). For further information on the EU novel foods authorisation process, see Novel foods explained.
Resources
Implementing Regulation (EU) 2017/2470 establishing the Union list of novel foods
Commission Implementing Regulation (EU) 2022/966 amending Implementing Regulation (EU) 2017/2470 as regards the conditions of use, the specific labelling requirements and specifications of the novel food Calanus finmarchicus oil
Regulation (EU) 2015/2283 on novel foods
Sources
Regulation (EU) 2025/1513 as regards the conditions of use and the additional labelling requirements of the novel food Calanus finmarchicus oil
Tables & Figures
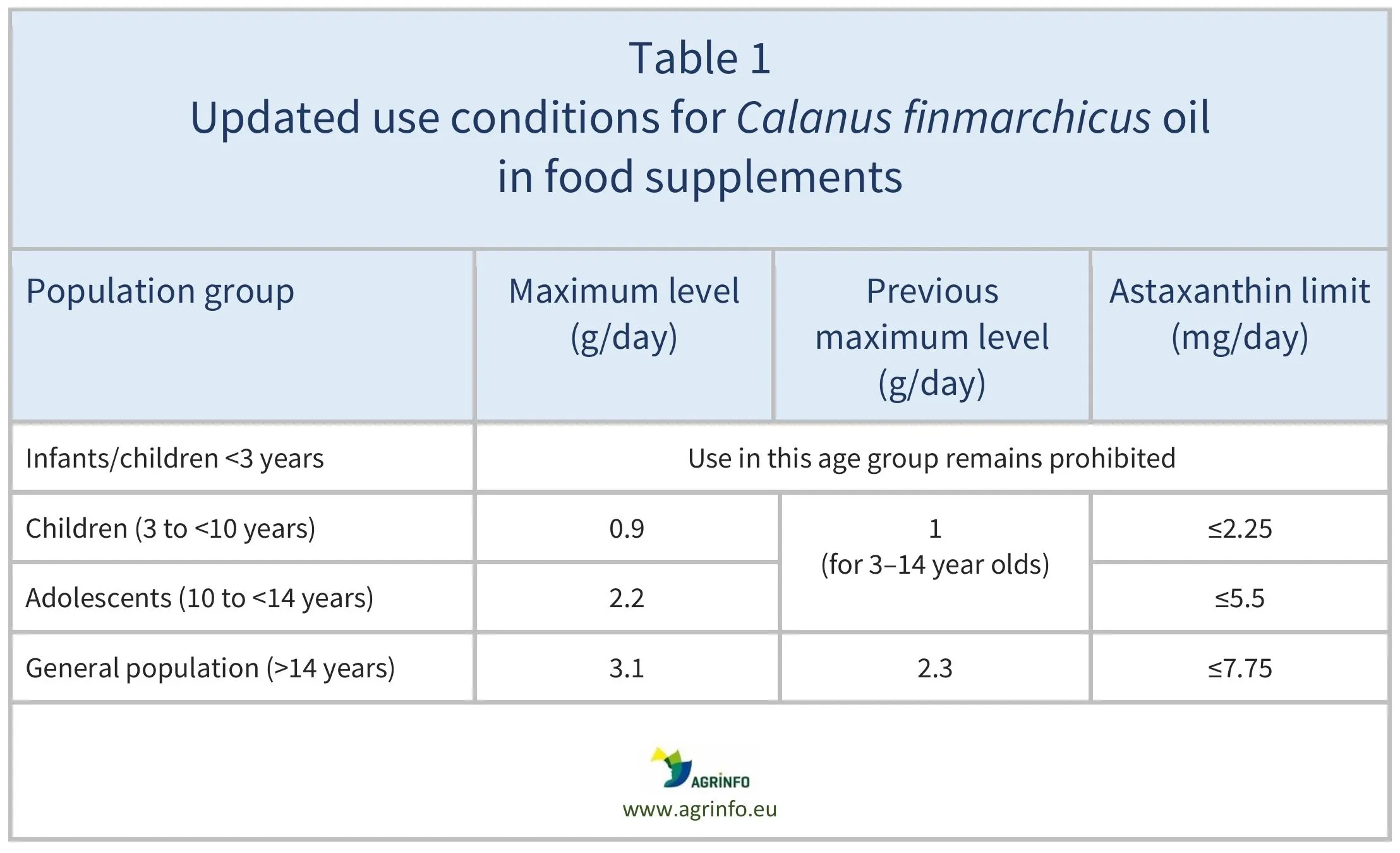
Source: based on Regulation 2025/1513
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
European Commission updates specification for novel food Calanus finmarchicus oil
Commission Implementing Regulation (EU) 2025/1513 as regards the conditions of use and the additional labelling requirements of the novel food Calanus finmarchicus oil
What is changing and why?
Calanus finmarchicus oil was originally authorised under Commission Implementing Decision 2017/2353. The specifications for this novel food (maximum levels and astaxanthin limits) have now been updated (see Table 1).
The labelling requirements have also been updated to reflect the new permitted limits.
Timeline
The new specification applies from 18 August 2025. Food supplements containing Calanus oil on the market or on their way to the EU before 18 August may be sold until their expiry date, provided they comply with Regulation 2017/2470, which was in place prior to these changes.
Tables & Figures

Source: based on Regulation 2025/1513
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
