Novel food: potassium magnesium trichloride hexahydrate
- Food safety
- Novel/traditional foods
Summary
The European Union (EU) has authorised the use of the novel food potassium magnesium trichloride hexahydrate in certain products intended for the adult population.
EU authorises use of novel food potassium magnesium trichloride hexahydrate in certain products
Commission Implementing Regulation (EU) 2025/1530 of 30 July 2025 authorising the placing on the market of potassium magnesium trichloride hexahydrate as a novel food and amending Implementing Regulation (EU) 2017/2470
Update
The European Union (EU) has authorised the use of the novel food potassium magnesium trichloride hexahydrate in certain products intended for the adult population.
Impacted Products
Raw and cooked cured (or seasoned) meat, cereal-based dishes, preserved sausages
What is changing?
The EU has authorised the use of potassium magnesium trichloride hexahydrate as a novel food in the following products intended for the adult population (excluding infants and children): meat, preserved sausages, and cereal-based dishes.
Where products contain potassium magnesium trichloride hexahydrate, they must:
- include potassium magnesium mineral salt in their labelling
- specify the amount present in the nutritional declaration where the amount of potassium and/or magnesium is significant (see Table 1).
Only the company that applied for authorisation, BK Giulini GmbH, is authorised to sell products containing this novel food on the EU market over the next 5 years, unless BK Giulini GmbH permits marketing by other companies, or if another company obtains a novel food authorisation without making reference to scientific data protected by BK Giulini GmbH.
Why?
The European Food Safety Authority (EFSA 2025) has concluded that the novel food potassium magnesium trichloride hexahydrate is safe when used in products intended for the adult population.
Timeline
This novel food may be placed on the EU market from 20 August 2025.
Background
This Regulation updates the Annex to Regulation 2017/2470 which lists authorised novel foods (see the Union list of novel foods). For further information on the EU novel foods authorisation process, see Novel foods explained.
Resources
EFSA (2025) Safety of mineral salt containing potassium and magnesium as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal, 23(1): e9205.
Regulation 1169/2011 on the provision of food information to consumers
Regulation 2017/2470 establishing the Union list of novel foods
Regulation 2015/2283 on novel foods
Sources
Commission Implementing Regulation (EU) 2025/1530 authorising the placing on the market of potassium magnesium trichloride hexahydrate as a novel food and amending Implementing Regulation (EU) 2017/2470
Tables & Figures
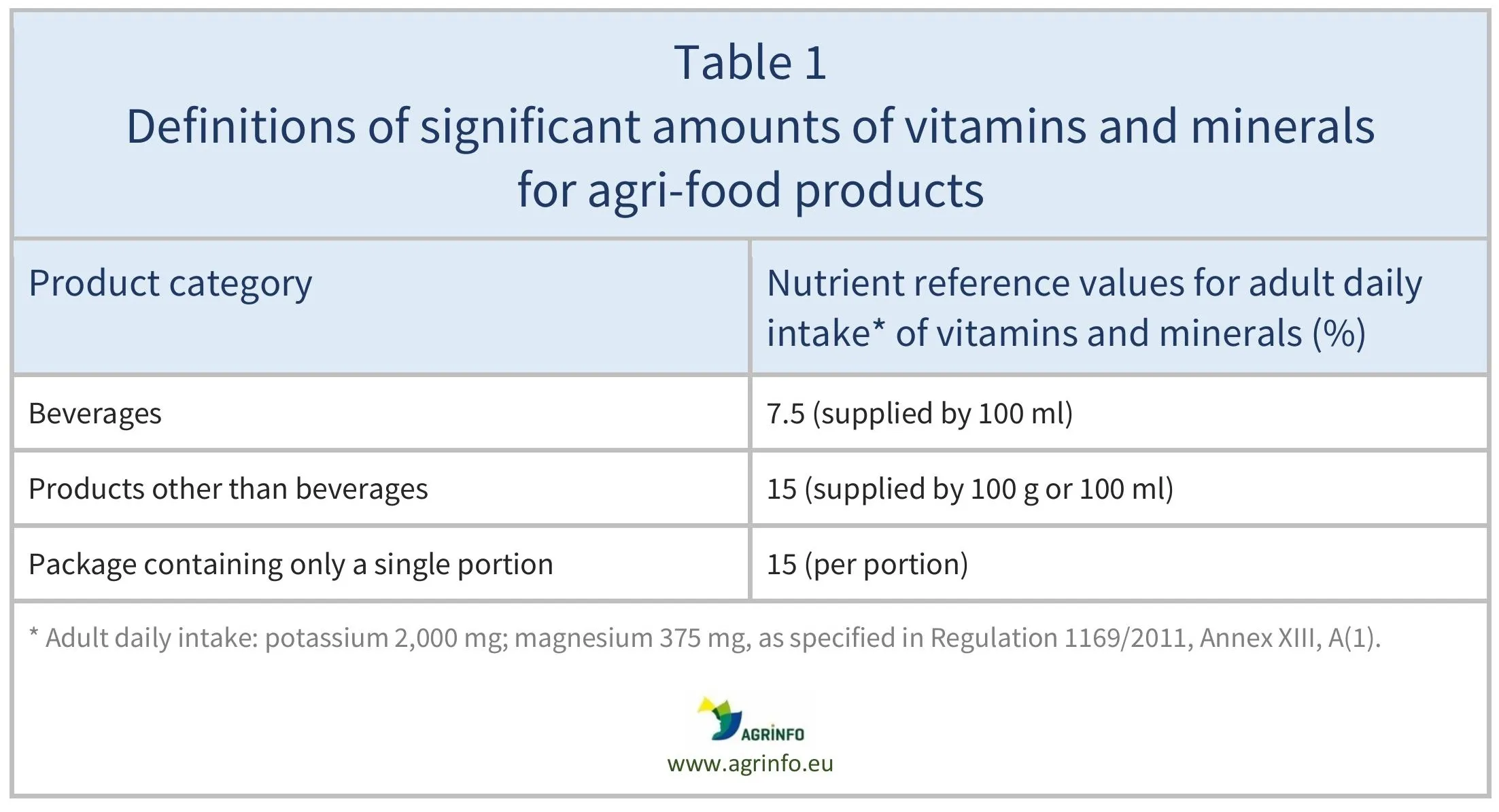
Source: based on Regulation 1169/2011, Annex XIII, A(1)
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU authorises use of novel food potassium magnesium trichloride hexahydrate in certain products
Commission Implementing Regulation (EU) 2025/1530 authorising the placing on the market of potassium magnesium trichloride hexahydrate as a novel food and amending Implementing Regulation (EU) 2017/2470
What is changing and why?
The European Union (EU) has authorised the use of the novel food potassium magnesium trichloride hexahydrate in meat, preserved sausages, and cereal-based dishes intended for the adult population. Specific labelling requirements apply.
Only the company that applied for authorisation, BK Giulini GmbH, is authorised to sell products containing this novel food on the EU market over the next 5 years, unless BK Giulini GmbH permits marketing by other companies, or if another company obtains a novel food authorisation without making reference to scientific data protected by BK Giulini GmbH.
Timeline
This novel food may be placed on the EU market from 20 August 2025.
Tables & Figures

Source: based on Regulation 1169/2011, Annex XIII, A(1)
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
