Novel food: Schizochytrium oil
- Food safety
- Novel/traditional foods
Summary
In November 2025, the European Union (EU) authorised new conditions of use for oil produced from Schizochytrium sp. strain ATCC PTA-9695 in food supplements intended for the general population.
This follows the previous authorisation of oil produced from Schizochytrium limacinum strain ATCC-20889 as a novel food for use in infant/follow-on formula (July 2025); and authorisation of new conditions of use for oil produced from Schizochytrium sp. strain FCC-3204 in protein products, excluding dairy analogues (April 2025).
EU authorises new conditions of use for Schizochytrium oil in food supplements
Commission Implementing Regulation (EU) 2025/2245 of 7 November 2025 amending Implementing Regulation (EU) 2017/2470 as regards the conditions of use of the novel food Schizochytrium sp. (ATCC PTA-9695) oil
Commission Implementing Regulation (EU) 2025/1515 of 28 July 2025 authorising the placing on the market of Schizochytrium limacinum (ATCC-20889) oil as a novel food and amending Implementing Regulation (EU) 2017/2470
Commission Implementing Regulation (EU) 2025/688 of 9 April 2025 amending Implementing Regulation (EU) 2017/2470 as regards the conditions of use of the novel food Schizochytrium sp. (FCC-3204) oil
Update
In November 2025, the European Union (EU) authorised new conditions of use for oil produced from Schizochytrium sp. strain ATCC PTA-9695 in food supplements intended for the general population.
This follows the previous authorisation of oil produced from Schizochytrium limacinum strain ATCC-20889 as a novel food for use in infant/follow-on formula (July 2025); and authorisation of new conditions of use for oil produced from Schizochytrium sp. strain FCC-3204 in protein products, excluding dairy analogues (April 2025).
Impacted Products
Protein products (excluding dairy analogues), infant nutrition, supplements
What is changing?
In November 2025, the EU authorised an increase in the maximum level of docosahexaenoic acid (DHA) permitted in food supplements for the general population (above 3 years of age) containing the novel food Schizochytrium sp. (ATCC PTA-9695) oil.
Earlier in 2025, the EU authorised:
- Schizochytrium limacinum ATCC-20889 as a novel food for use in infant/follow-on formula (Regulation 2025/1515)
- new conditions of use for the novel food Schizochytrium sp. FCC-3204 in protein products intended for the general population, excluding dairy analogues (products using plant-based ingredients that mimic dairy products) (Regulation 2025/688).
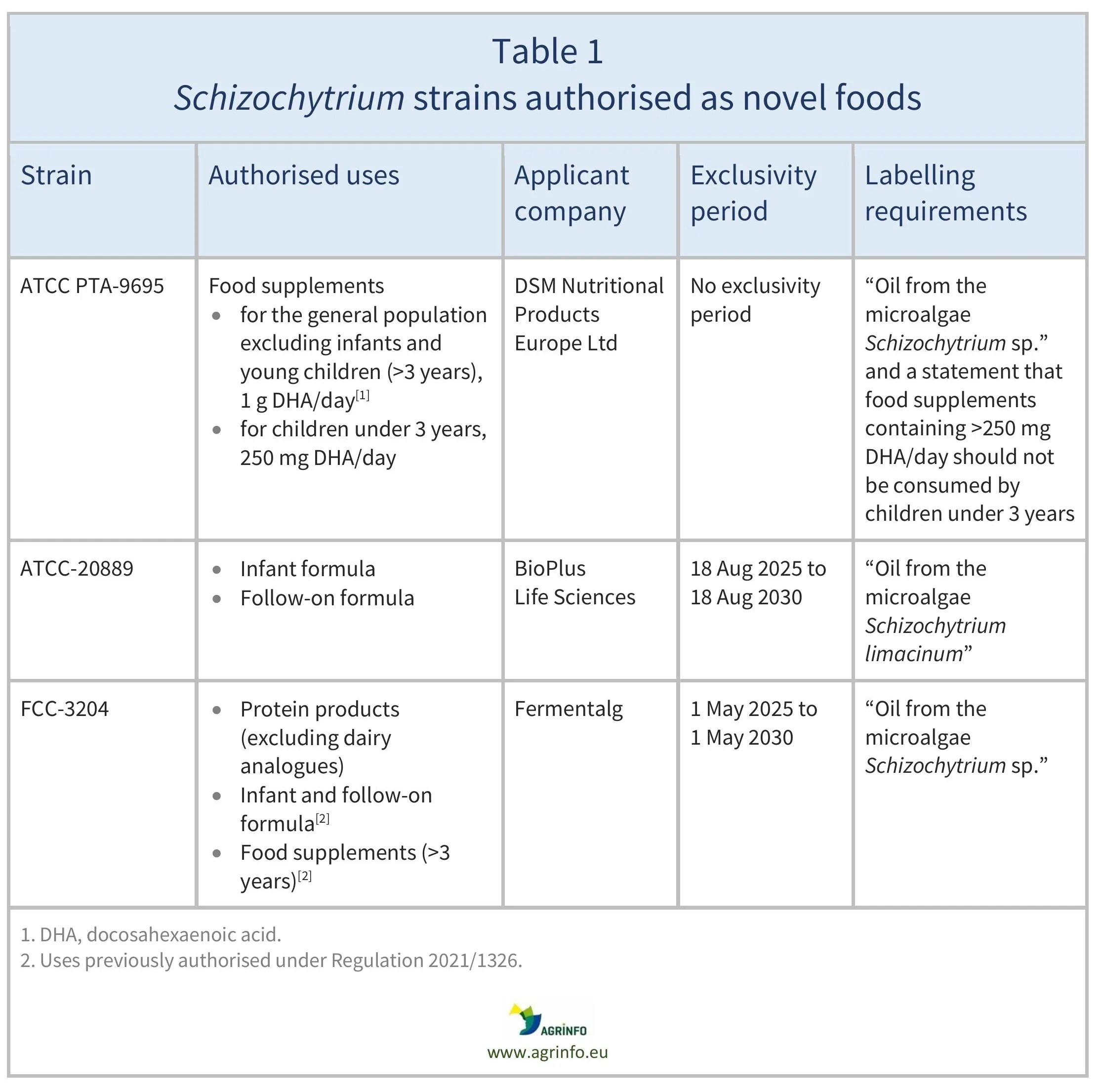
Table 1 provides details of the strains, authorised uses, and companies that requested authorisation.
Only the companies that applied for the authorisations of Schizochytrium sp. FCC-3204 in protein products and S. limacinum (ATCC-20889) as a novel food for use in infant/follow-on formula may market these novel foods for the uses authorised for a 5-year period (see Table 1), unless these companies provide permission to others to market, or if another company obtains a novel food authorisation for this use without reference to scientific data protected in the novel food authorisations. There is no exclusivity period for the newly authorised conditions of use for Schizochytrium sp. (ATCC PTA-9695) oil.
Why?
On the basis of earlier evaluations by the European Food Safety Authority (EFSA 2012, 2021), the European Commission has concluded that the proposed increased levels of DHA in food supplements do not raise health concerns.
EFSA (2025) provided a positive safety evaluation of S. limacinum strain ATCC-20889.
EFSA (2024) concluded that the novel food Schizochytrium FCC-3204 oil is safe when used in protein products intended for the general population, excluding dairy analogues.
Timeline
New conditions for use of:
- Schizochytrium sp. (ATCC PTA-9695) oil in food supplements intended for the general population – apply from 30 November 2025
- Schizochytrium (FCC-3204) oil in protein products – apply from 1 May 2025.
The use of S. limacinum (ATCC-20889) oil in infant/follow-on formula is permitted from 18 August 2025.
Background
These Regulations update the Annex to Regulation 2017/2470 which lists authorised novel foods (see the Union list of novel foods). For further information on the EU novel foods authorisation process, see Novel foods explained.
Resources
EFSA (2012) Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA Journal, 10(7): 2815.
EFSA (2021) Safety of oil from Schizochytrium limacinum (strain FCC‐3204) for use in food supplements as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal, 19(1): 6345.
EFSA (2024) Safety of an extension of use of oil from Schizochytrium limacinum (strain FCC-3204) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal, 22: e9043.
EFSA (2025) Safety of oil from Schizochytrium limacinum (strain ATCC-20889) for use in infant and follow-on formula as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal, 23(1): e9156.
Regulation 2021/1326 authorising the placing on the market of Schizochytrium sp. (FCC-3204) oil as a novel food.
Regulation 2017/2470 establishing the Union list of novel foods.
Regulation 2015/2283 on novel foods.
Sources
Commission Implementing Regulation (EU) 2025/2245 as regards the conditions of use of the novel food Schizochytrium sp. (ATCC PTA-9695) oil
Commission Implementing Regulation (EU) 2025/1515 authorising the placing on the market of Schizochytrium limacinum (ATCC-20889) oil as a novel food
Commission Implementing Regulation (EU) 2025/688 as regards the conditions of use of the novel food Schizochytrium sp. (FCC-3204) oil
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU authorises new conditions of use for Schizochytrium oil in food supplements
Commission Implementing Regulation (EU) 2025/2245 as regards the conditions of use of the novel food Schizochytrium sp. (ATCC PTA-9695) oil
Commission Implementing Regulation (EU) 2025/1515 authorising the placing on the market of Schizochytrium limacinum (ATCC-20889) oil as a novel food
Commission Implementing Regulation (EU) 2025/688 as regards the conditions of use of the novel food Schizochytrium sp. (FCC-3204) oil
What is changing and why?
The European Union (EU) has authorised the use of the novel food Schizochytrium sp. (ATCC PTA-9695) oil in food supplements intended for the general population, allowing increased levels of docosahexaenoic acid (DHA).
This follows the authorisation earlier in 2025 of:
- Schizochytrium limacinum (ATCC-20889) as a novel food for use in infant/follow-on formula
- new conditions of use for the novel food Schizochytrium sp. (FCC-3204) oil in protein products.
Table 1 provides details of the strains, authorised uses, and companies that sought these authorisations.
Timeline
New conditions for use of:
- Schizochytrium sp. (ATCC PTA-9695) oil in food supplements intended for the general population – apply from 30 November 2025
- Schizochytrium sp. (FCC-3204) oil in protein products – apply from 1 May 2025.
The use of S. limacinum (ATCC-20889) oil in infant/follow-on formula is permitted from 18 August 2025.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.