Novel foods explained
- Novel/traditional foods
Summary
Background information on the rules on bringing to the EU market novel foods, and foods traditionally eaten outside Europe but sold for the first time in the EU. Legal definitions of novel and traditional, and the procedures and basic requirements, are explained.
Background information on the rules for bringing to the EU market novel foods, and foods traditionally eaten outside Europe but sold for the first time in the EU
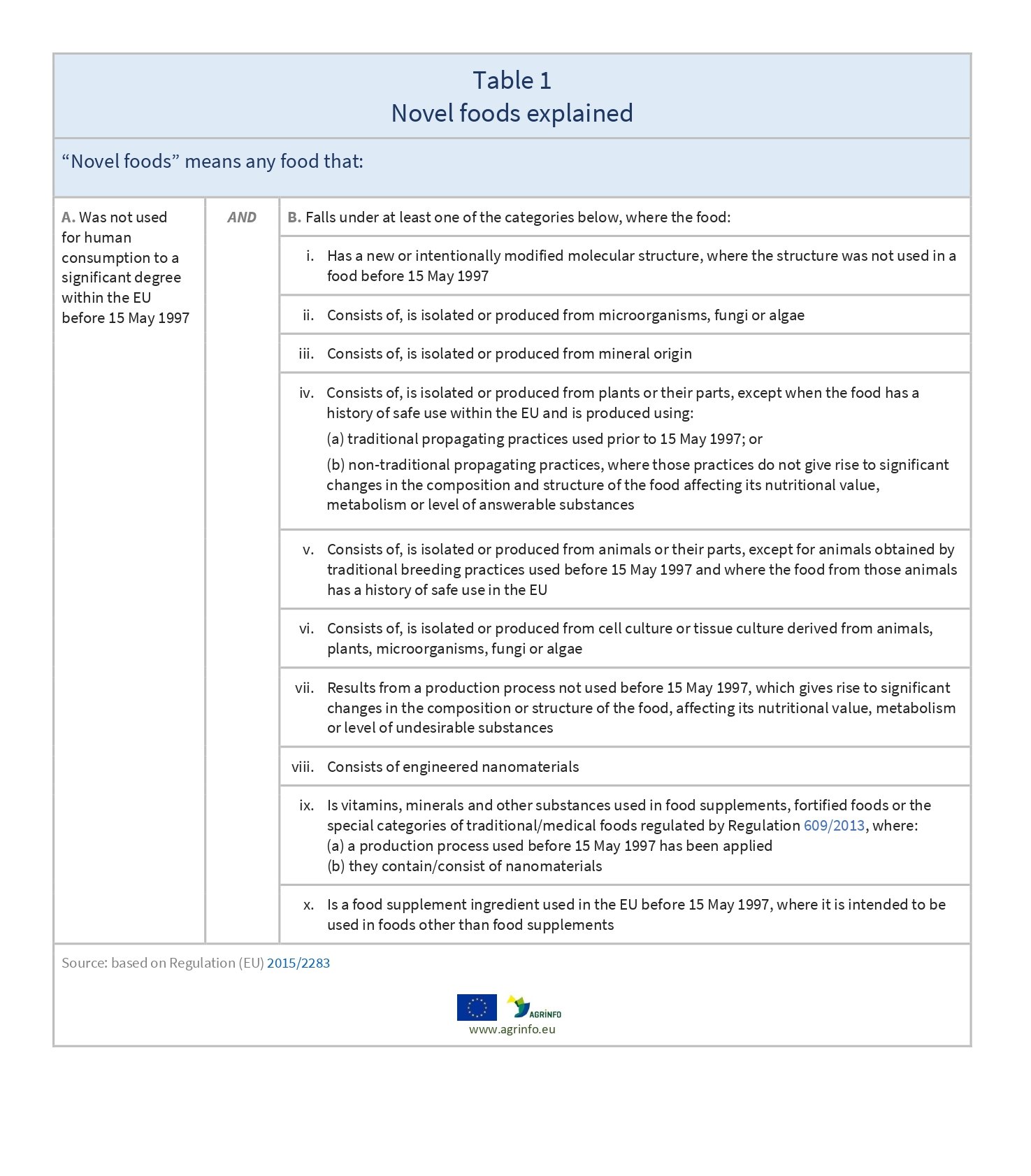
Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001
Update
Background information on the rules on bringing to the EU market novel foods, and foods traditionally eaten outside Europe but sold for the first time in the EU. Legal definitions of novel and traditional, and the procedures and basic requirements, are explained.
Background
Since 1997, the EU has had a specific regulatory procedure for all novel foods – that is food that was not on the European market before 1997. Regulation 258/97 was replaced by Regulation (EU) 2015/2283, which aimed to clarify and improve the efficiency of the authorisation procedures. Of particular relevance for agri-food exporters to the EU was the introduction of a simplified procedure for “traditional food” historically consumed safely in third countries. Novel foods, including traditional foods, are regularly approved for placement on the EU market and included in a Union list of novel foods (Regulation (EU) 2017/2470).
NOVEL FOODS
What is a novel food?
A food is determined to be novel where it fulfils two criteria, as shown in Table 1.
What happens if the food status – novel or not novel – is not clear?
If a product’s novel food status has already been discussed by EU Member States, information about its perceived status can be found in the EU Novel food catalogue. This online resource indicates whether a food is novel; not novel; historically only used in food supplements (and therefore novel); or if there is insufficient information to decide. This catalogue has no legal value; it provides an indicative view on the basis of information collected by Member States.
If the novel food status is still unclear, a food business can consult with the authorities of the Member State where they first intend to place a product on the market. Those authorities can in turn consult with other Member States and the Commission. A final decision on the status of the food can be made by the Commission. A list of past consultations can be found on the European Commission webpage “Consultation process on novel food status”. Up to 2022, in cases where status clarification was requested, around 60% of the foods were considered novel.
How can a business get a novel food on the authorised Union list of novel foods?
A business can apply for a novel food authorisation by providing the following information to the Commission (Regulation (EU) 2015/2283, Art. 10):
- name/address of applicant
- name/description of novel food
- description of production process
- detailed composition of novel food
- scientific evidence demonstrating the food does not pose a safety risk to human health
- analysis methods (where appropriate)
- proposal for the intended use and specific labelling requirements.
Applications must be submitted to the European Commission using the e-submission system
How is the authorisation application handled?
The European Commission may, and typically does, request an opinion from the European Food Safety Authority (EFSA) on the safety elements of the application (Art. 10). EFSA must provide its opinion within 9 months on:
- the novel food’s safety compared to comparable food on the EU market
- whether the novel food poses a safety risk to human health
- whether the novel food, if it is intended to replace another food, could be nutritionally disadvantageous for the consumer.
EFSA can ask for additional information from the applicant, and may request an extension to the 9-month evaluation period.
Having received EFSA’s opinion, the Commission has 7 months to submit to Member States a draft act authorising the product. If the Commission does not request an EFSA opinion, the 7-month period starts from the date when the Commission receives the application.
The Commission can terminate the application procedure at any stage and decide not to update the Union list of novel foods. A list of terminated novel food applications can be found on the European Commission webpage “Decisions terminating the procedure”.
TRADITIONAL FOODS
What if a food is not known in the EU, but is part of the traditional diet in a non-EU country?
A different procedure is available for traditional foods from third countries (Regulation (EU) 2015/2283, Art. 14). Traditional foods are those with a history of safe use in a third country, defined as having their safety “confirmed with compositional data and from experience of continued use for at least 25 years in the customary diet of a significant number of people in at least one third country” (Art. 3).
A business wishing to export a traditional food to the EU must notify the Commission with the following information:
- name/address of applicant
- name/description of traditional food
- detailed composition of traditional food
- country/countries of origin of traditional food
- data demonstrating the history of safe food use in a third country
- proposal for conditions of intended use/specific labelling requirements.
Further details of what is required in an application are set out in Commission Implementing Regulation 2017/2468.
How are traditional food notifications handled?
The Commission forwards notifications to EFSA and the Member States. If, within 4 months, it receives no substantiated safety objections concerning the traditional food, the Commission must authorise its placing on the market. If safety objections are received, the business must make an application including data that responds to the objections raised.
Guidance for traditional foods
- Commission Implementing Regulation 2017/2468 details the administrative and scientific requirements for notifications concerning traditional foods
- EFSA: Notification procedure for traditional foods
- EFSA: Guidance on the preparation and submission of the notification and application for authorisation of traditional foods from third countries in the context of Regulation (EU) 2015/2283 (Revision 1)
- A food business wishing to market a traditional food from a third country for the first time must submit a notification directly to the European Commission using the e-submission system.
What are the major implications for exporting countries?
Opportunities
The EU offers a significant market for developing countries commercialising biological resources beyond their borders, and the Novel Foods Regulation provides a secure legal basis for such exports. For example, with the support of UNCTAD, South Africa secured novel food status for baobab fruit pulp in the EU. Exports to the EU have contributed to a global baobab ingredient market estimated at $3.8 billion in 2017, which is expected to expand to $5 billion by 2024. Baobab commercialisation, although initiated in South Africa, offers economic opportunities to several African countries (UNCTAD 2021). In the case of baobab, EU authorisation also paved the way to regulatory approval in other international markets, including Canada, Australia, Japan, South Korea, India, Malaysia, Singapore and Thailand (ABioSA 2021). Extending use of the byproducts of traditional ingredients, such as coffee byproducts (recognised by the EU as novel foods), is also a way of reducing waste and providing additional income for farmers (Lachenmeier et al. 2021).
Limitations
The EU Novel Foods Regulation has long been controversial among many developing countries, some of which have raised concerns before the World Trade Organization (WTO). Peru, for example, has been consistently critical of the treatment of "high potential Peruvian products such as camu camu (Myrciaria dubia), yacón (Smallanthus sonchifolius), sacha inchi (Plukenetia volubilis) and other Amazonian fruits and their by-products" as novel foods (WTO 2011).
The 2015 revision of the regulation partly aimed to facilitate market access to traditional products. However, its success in this respect is questionable. By 2022, 16 notifications of traditional foods from non-EU countries had been submitted to the European Commission, and eight had been included on the Union list. The limited number of traditional food notifications is particularly notable given the large increase in novel food applications, up from an average of five per year before 2015 to 40 applications in 2018 and 39 in 2019 (Ververis et al. 2020). Market expansion for traditional foods has therefore been limited.
One potential obstacle may be the cost of gathering the data required to support notifications and applications. For example, for baobab the process is estimated to have cost between €250,000 and €350,000 (albeit under the Directive that preceded this Regulation) (UNCTAD 2021). Third countries have continued to raise questions before the WTO regarding the scientific justification for the establishment of an approval/notification procedure for traditional foods (WTO 2017; WTO 2019).
Resources
ABioSA (2021) How the baobab industry developed: From emerging to maturing sector.
Lachenmeier, D.W. et al. (2021) An update on sustainable valorization of coffee by-products as novel foods within the European Union. Biology and Life Sciences Forum, 6(1): 37.
UNCTAD (2021) Implications of the African Continental Free Trade Area for trade and biodiversity: Policy and regulatory recommendations.
Ververis, E. et al. (2020) Novel foods in the European Union: Scientific requirements and challenges of the risk assessment process by the European Food Safety Authority. Food Research International, 137: 109515.
WTO (2011) Regulation 258/97 of the European Parliament and of the Council concerning novel foods: Communication from Peru. G/SPS/GEN/1087.
WTO (2017) Committee on Sanitary and Phytosanitary Measures: Summary of the meeting of 22–23 March 2017. G/SPS/R/86.
WTO (2019) Trade Policy Review. WT/TPR/S/395.
Sources
Regulation (EU) 2015/2283
Tables & Figures
.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
