Official controls of contaminants in foods
- Contaminants
Summary
The EU has adopted two new Regulations on uniform controls of contaminants in food of animal origin and composite products, which include minimum control frequencies and a risk-based approach. Countries exporting to the EU must put in place an equivalent risk-based control plan to be approved by the European Commission.
EU publishes Regulations to standardise official controls of contaminants in food of animal origin and composite products
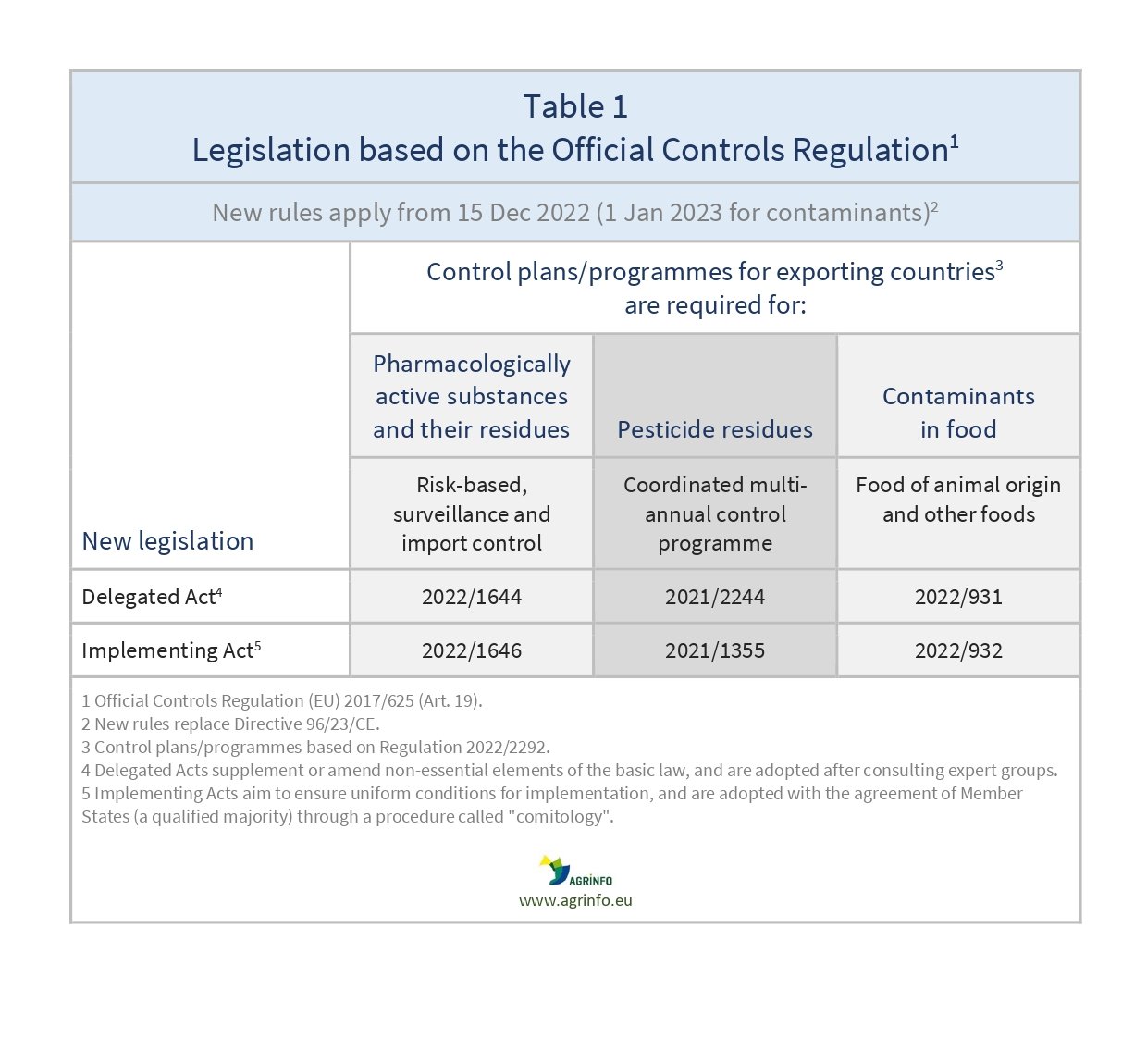
Delegated Regulation 2022/931 supplementing Regulation 2017/625 by laying down rules for the performance of official controls as regards contaminants in food
Implementing Regulation 2022/932 on uniform practical arrangements for the performance of official controls as regards contaminants in food
Update
The EU has adopted two new Regulations on uniform controls of contaminants in food of animal origin and composite products, which include minimum control frequencies and a risk-based approach. Countries exporting to the EU must put in place an equivalent risk-based control plan to be approved by the European Commission.
Impacted Products
meat, offal, fish, crustaceans, dairy, eggs, honey, insects
What is changing?
Regulations 2022/931 and 2022/932 replace Directive 96/23/EC to provide specific rules for official controls of contaminants in animal products and composite products. Competent authorities must submit a risk-based control plan to the Commission that demonstrates how they comply with EU legislation for contaminants in food.
In addition to contaminants, control plans cover residues of pharmacologically active substances, and residues of pesticides (Regulation 2021/1355).
Controls of commodities from exporting countries to the EU take place at two levels:
- by exporting countries themselves
- by the Member State authorities at the border control post of the importing European country.
Exporting countries’ control plans for foods of animal origin must provide guarantees of controls at least equivalent to those required from EU Member States (Regulation 2022/2292, Art. 12).
Control plans must include information on:
- the combinations of contaminants or contaminant groups and commodity groups to be controlled (2022/931, Annex I)
- the sampling strategy (when, where, what), including any history of non-compliance, especially cases reported under the Rapid Alert System for Food and Feed (RASFF) (2022/931, Annex II)
- the actual control frequencies for food placed on the EU market, taking into account the minimum control frequencies (2022/932, Annexes I and II).
Exporting countries must justify the content of control plans with reference to risk analysis.
A control plan can cover the total national production, or only production eligible for export to the EU (for example, it could be limited to establishments that export to the EU market). For this second option, the plan must describe how EU-compliant production is segregated (2022/2292, Annex I, Part II, A4).
The European Commission updated its Guidelines on control plans in February 2023.
Guidance and templates for controls by Member States at border control posts are available via the Commission's Sampling and Analysis webpage.
Why?
Directive 96/23/EC, which included EU obligations to monitor contaminants, was repealed on 14 December 2022. These two new Regulations update and adapt the Official Controls Regulation, ensuring continued controls of contaminants in food.
Timeline
Date of publication: 17 June 2022.
Date of entry into application: 1 January 2023.
What are the major implications for exporting countries?
Listing
Exporting countries can only export food of animal origin to the EU if they are included in the European Commission's Establishment Lists as authorised to do so. The control of contaminants in food of animal origin, in compliance with Regulations 2022/931 and 2022/932, is a requirement in order to be included in the list. Exporting countries must submit their control plan and provide guarantees of compliance with contaminant maximum levels. The level of controls in the exporting country must be at least equivalent to that required from EU Member States for EU production.
Fishery products
For countries exporting fish, the control plan also includes official controls on fishery products which are carried out on vessels calling at a port in a Member State.
Submission of control plans
The control plan must be submitted by 31 March each year. For plans due by 31 March 2024, the updated templates must be used (European Commission 2023, 4.4).
The Excel template is comprehensive, thus more complicated than before. For ease of use, exporting countries are advised to delete the sections that are not relevant to them. The template covers residues of pharmacologically active substances, pesticides and contaminants under different tabs. Tabs (a) to (e) provide additional information and guidance.
Exempted products
Gelatine, collagen, and raw materials for their production, highly refined products of animal origin, insects, frogs, frogs’ legs, snails, reptiles and reptile meat are excluded from the scope (2022/2292, Art. 5.2). No control plan is necessary for these products.
Recommended Actions
Countries that export food of animal origin and composite products to the EU must ensure controls of contaminants, equivalent to those for EU Member States. This is a public health obligation required in order to be included in the list of countries authorised to export these products to the EU (Regulation 2022/2292, Art. 6.2 (see Third country lists and Public health requirements).
Control plans must be risk-based. Competent authorities must justify their sampling strategy according to the required minimum sampling frequency.
EU Member States will look for possible non-compliances when checking commodities at border control posts.
Competent authorities in exporting countries can send any questions on this topic to the European Commission by emailing sante-tcresidueplans@ec.europa.eu. They can also ask the European Union Delegation in their country to organise training.
Background
Control plans
The Official Controls Regulation (2017/625) establishes rules for official controls of both production within the EU, and products exported to the EU.
Delegated Regulation (EU) 2022/2292 supplements this basic Regulation regarding specific public health requirements for food-producing animals and animal products exported to the EU, replacing Directive 96/23 (see Table 1).
Maximum levels
Regulation (EU) 2023/915 setting the maximum levels of contaminants in food complements these Regulations. For certain specific products, maximum levels are set in other Regulations (European Commission 2022, section 10).
The Commission's updated Guidelines on control plans (2023) explain all the requirements for animal products in one document.
Resources
European Commission (2022) Guidance on the implementation of the rules and practical arrangements for the performance of the official controls as regards contaminants in food.
European Commission (2023) Guidelines on EU requirements for imports of products of animal origin – Control plans for residues of veterinary medicines, pesticides and contaminants.
European Commission: Official controls on imported products
Commission Notice on a guidance document on the implementation of the requirements for the multi-annual national control plans as set out in Articles 109 to 111 of Regulation (EU) 2017/625.
Additional resources for Table 1:
Sources
Delegated Regulation 2022/2292 with regard to requirements for the entry into the Union of consignments of food-producing animals and certain goods intended for human consumption
Delegated Regulation 2022/931 laying down rules for the performance of official controls as regards contaminants in food
Implementing Regulation 2022/932 on uniform practical arrangements for the performance of official controls as regards contaminants in food
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
