Official controls on the use of pharmacologically active substances and their residues
- Veterinary residues
Summary
The EU has published new Regulations on official controls of residues of pharmacologically active substances in food-producing animals and animal products. Exporting countries must submit their risk-based control plan, giving guarantees at least equivalent to those for food produced in the EU, and taking into account the minimum sampling frequency requirements.
Uniform rules on national control plans for residues of pharmacologically active products in food-producing animals and animal products
Commission Implementing Regulation (EU) 2022/1646 of 23 September 2022 on uniform practical arrangements for the performance of official controls as regards the use of pharmacologically active substances authorised as veterinary medicinal products or as feed additives and of prohibited or unauthorised pharmacologically active substances and residues thereof, on specific content of multi-annual national control plans and specific arrangements for their preparation
Update
The EU has published new Regulations on official controls of residues of pharmacologically active substances in food-producing animals and animal products. Exporting countries must submit their risk-based control plan, giving guarantees at least equivalent to those for food produced in the EU, and taking into account the minimum sampling frequency requirements.
Impacted Products
cattle, sheep, goat, pigs, horses, aquaculture, rabbit, farmed game, reptiles, insects, meat, edible offal, casings, eggs, milk, dairy, honey
What is changing?
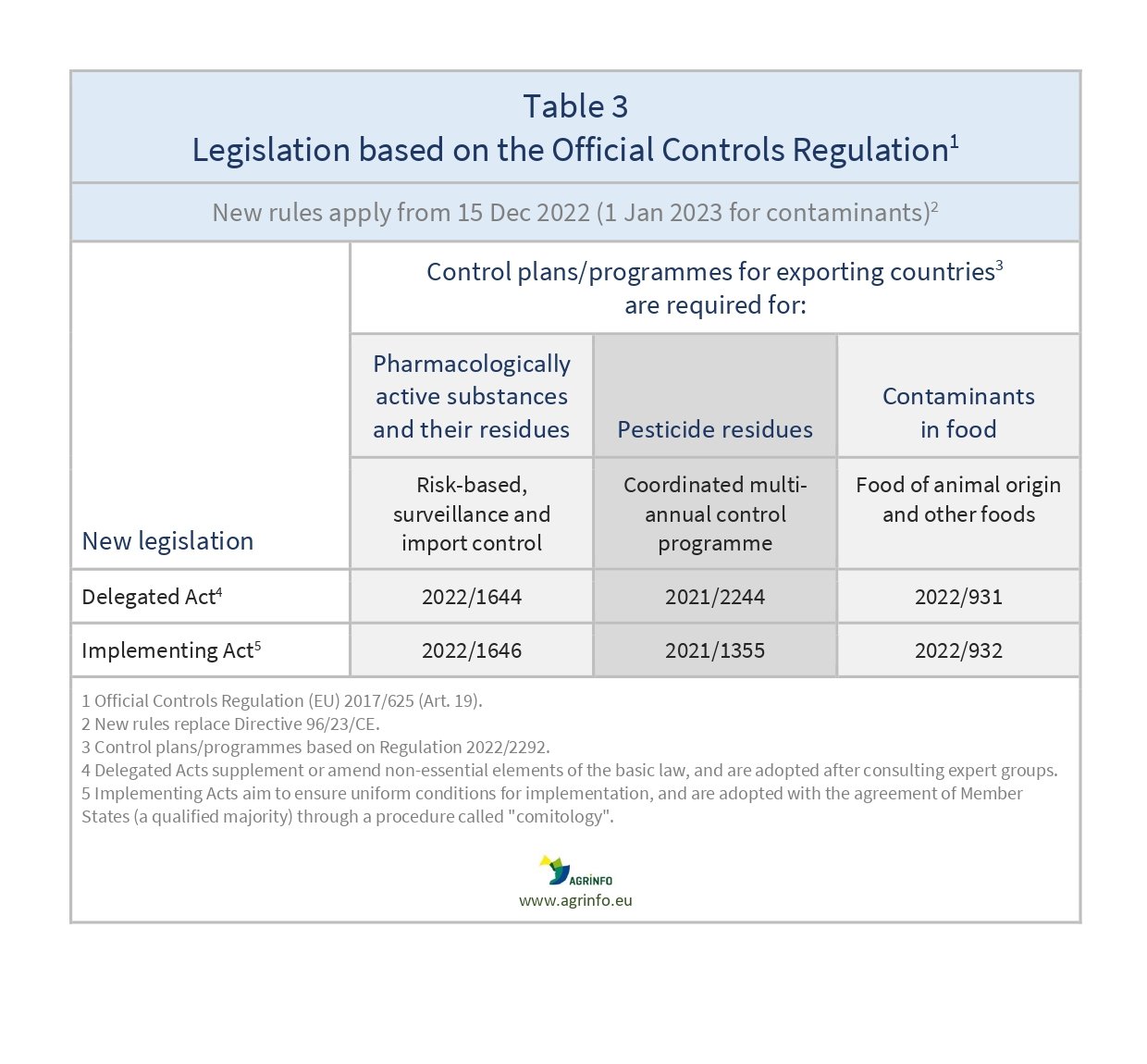
Two new Regulations, (EU) 2022/1646 and (EU) 2022/1644, together replace the Official Controls Directive 96/23/EC on the use of pharmacologically active substances authorised as veterinary medicinal products or as feed additives in live animals and animal products.
Controls of commodities from exporting countries to the EU take place at two levels:
- by exporting countries themselves
- by the Member State authorities at the border control post of the Member State.
Control plans in exporting countries
Exporting countries’ control plans for food of animal origin must be at least equivalent to those required from EU Member States (2022/2292, Art. 4 and 9). Controls must be based on:
- the combinations of substances and species, products in accordance with Regulation (EU) 2022/1644 (Annex II)
- the sampling strategy (Annex III)
- the actual sampling frequencies, taking into account the annual minimum control frequencies (Annex I)
- analytical methods to be used.
The new element is that the controls must be risk-based. Exporting countries must now explain their risk analysis to support the control plan they have adopted.
When a substance is not authorised in the EU, but is authorised in the exporting country, that country must demonstrate the absence of residues in the exported commodities (Regulation (EU) 2021/808, Annex I, or equivalent requirements).
When a substance forbidden in the EU is authorised in the exporting country, it must have a separate production system in place for its exports to the EU. It must also be able to guarantee compliance with strict conditions, including an appropriate animal identification and traceability system that is able to control distribution of the prohibited substances, and must maintain records of administration of veterinary medicinal products) (Delegated Regulation (EU) 2022/2292, Art. 10). Substances forbidden in the EU include beta-agonists; any stilbene, thyrostatic, oestrogenic, androgenic or gestagenic substances; chloramphenicol; nitrofurans.
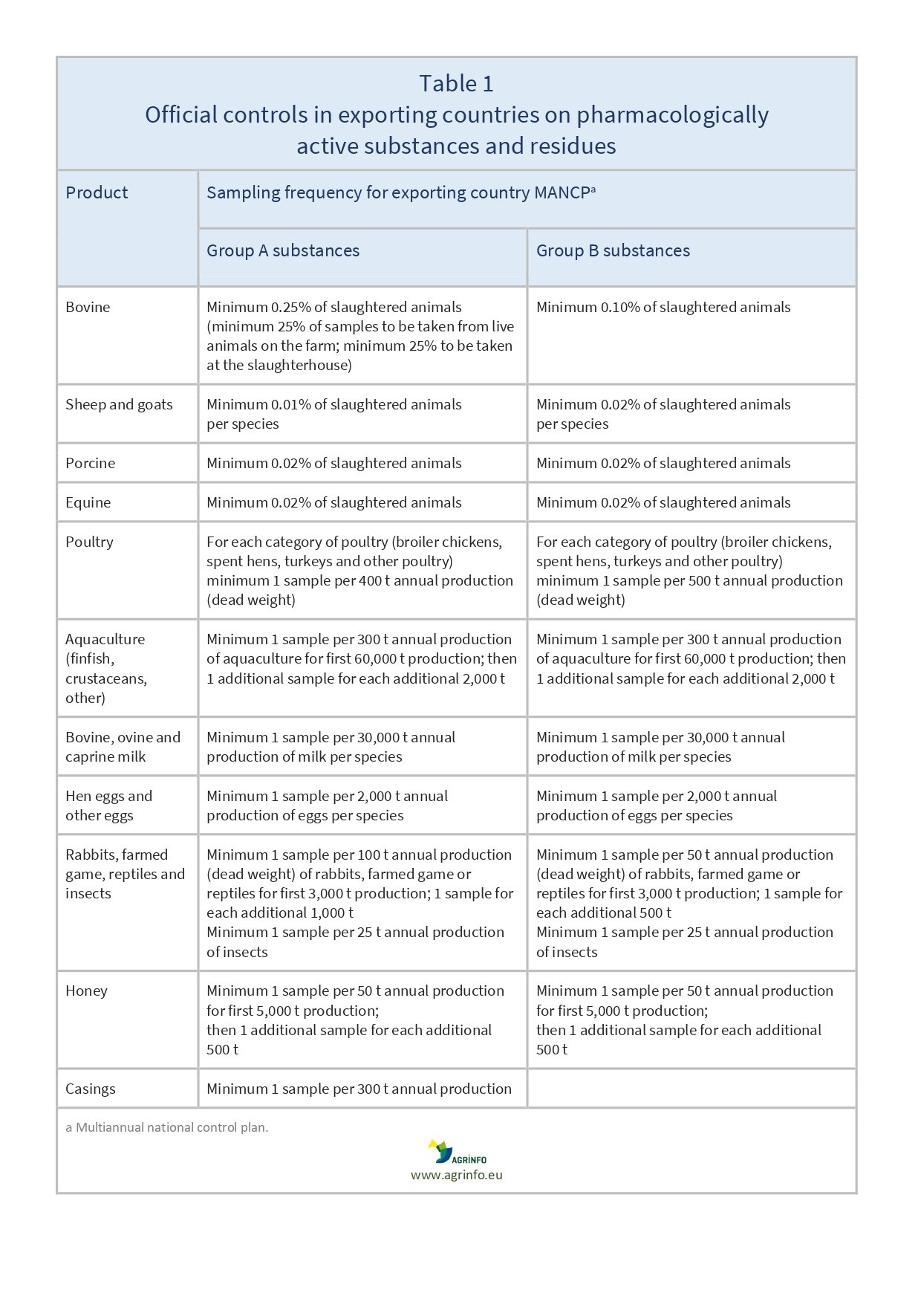
The minimum sampling frequencies are set out in 2022/1646, Annex I (see Table 1). The control plan can cover the total national production, or only production eligible for export to the EU. For the latter option, a description of the system in place is needed so that only animals and products from this controlled production can be exported to the EU.
For composite products, a control plan is not needed if the components of animal origin originate from a Member State or an exporting country with an approved control plan for food-producing animals or products of animal origin. If that is the case, the exporting country must detail the procedures in place to guarantee traceability from that origin (2022/2292, Art. 8).
Controls at the EU border
EU Member States must have a national risk-based control plan for third country imports (Art. 6).
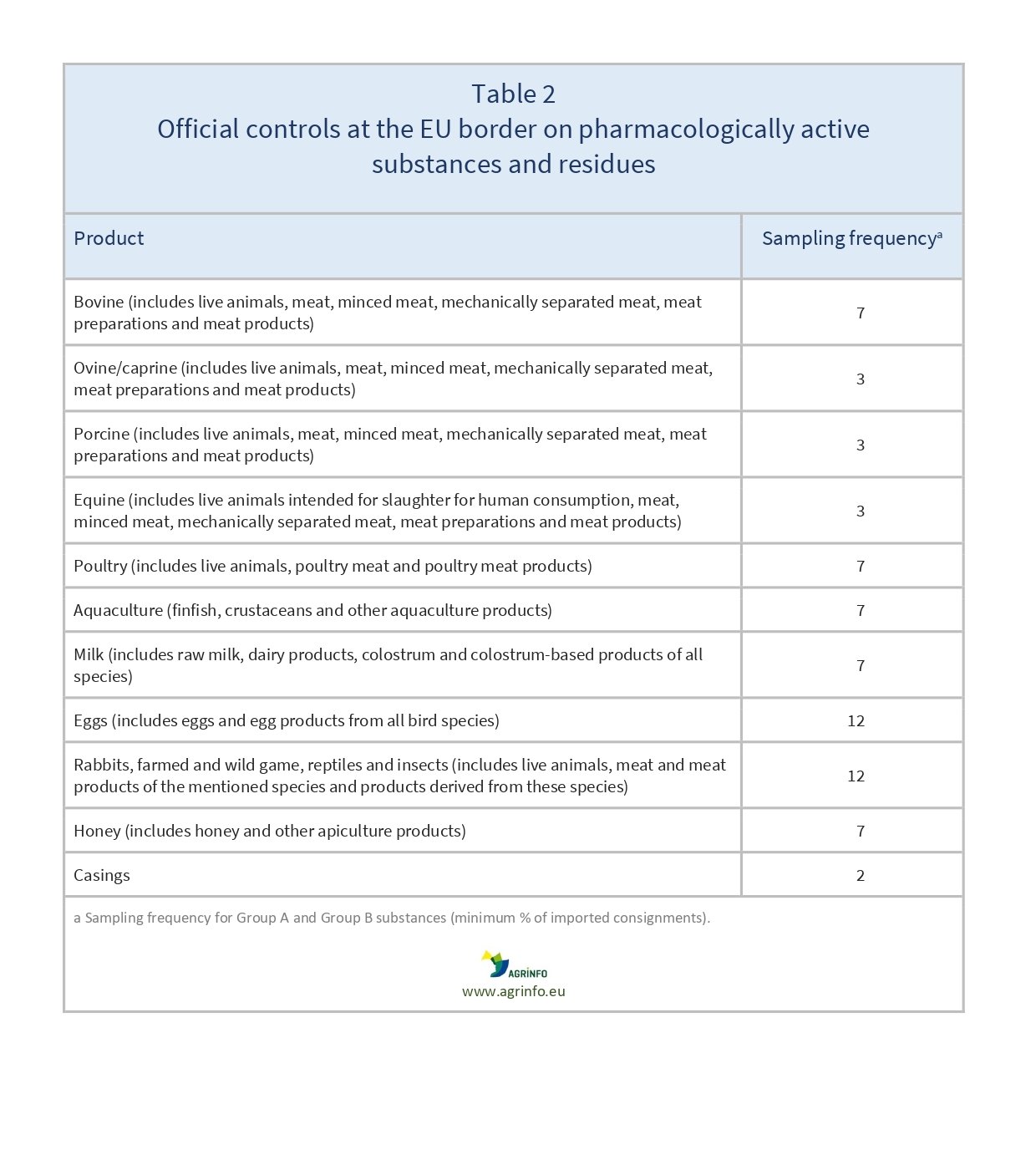
The required minimum sampling frequency is set out in Implementing Regulation (EU) 2022/1646 (Annex III) (see Table 2). This covers all farmed food-producing animals and their products (mainly unprocessed), including horses and casings.
The European Commission updated its Guidelines on control plans in May 2023.
Why?
Directive 96/23/EC and Decision 97/747/EC were repealed on 14 December 2022 and replaced by Regulation (EU) 2022/1646 concerning:
- the content of the multiannual national control plan (MANCP) that Member States must develop, according to the Official Controls Regulation (2017/625) to ensure plans are harmonised, allowing data comparisons
- official controls of residues of substances with pharmacological action, the minimum sampling frequency of their metabolites, and of other substances transmissible to animal products that are likely to be harmful to human health.
Timeline
Date of publication: 26 September 2022.
Date of application: 15 December 2022.
What are the major implications for exporting countries?
- As before, countries exporting animals and animal products to the EU must prepare their own control plan, ensuring that their exports are in compliance with the EU legislation. The new element is that controls must now be risk-based. The risk analysis must explain how the controls comply with the minimum frequencies (see Table 1).
- Regarding controls at EU border control posts, exports to the EU will be systematically checked according to the stated sampling frequency (see Table 2).
- Exporting countries that submit control plans giving guarantees equivalent to those of the Member States, validated by the European Commission, will be included in the list of countries authorised to export the relevant animals or animal products.
- No control plan is necessary for gelatine and collagen, and raw materials for their production; highly refined products of animal origin; insects; frogs, frogs’ legs; snails; reptiles and reptile meat (2022/2292, Art. 5.2).
Recommended Actions
Countries exporting bovine (cattle and beef), ovine (sheep, lamb, sheepmeat), caprine (goat and goatmeat), porcine (pigs and pork), equine (horses and horsemeat), aquaculture, poultry, milk, eggs, rabbit, wild game, honey, and casings must lay down annual control plans to monitor Group A and Group B substances.
These control plans must give the same guarantees as those of the Member States. The new element is that the control plans must be risk-based. This means that competent authorities must be able to justify their sampling strategy according to the minimum frequency (see Table 1).
Food-producing animals and animal products from exporting countries are systematically controlled at EU border control posts. To prevent non-compliance, the risk analysis determined in the control plan and required sampling frequency should rigorously follow the criteria in Table 2. EU Member States will consider any history of non-compliance, especially cases reported under the Rapid Alert System for Food and Feed (RASFF), when checking commodities at their border control posts. Controls are likely to be reinforced when non-compliances are detected.
Control plans must be submitted every year by 31 March. The updated templates (Guidelines on control plans, 4.4) must be used to report the 2023 results by 31 March 2024.
The Excel template is comprehensive, thus more complicated than before. For ease of use, exporting countries are advised to delete is the sections not relevant to them. The template covers residues of pharmacologically active substances, pesticides and contaminants under different tabs. Tabs (a) to (e) provide additional information and guidance.
Background
The Official Controls Regulation (EU 2017/625) foresees that competent authorities have to perform official controls, at any stage of production, processing and distribution, on residues of relevant substances in food and feed (including substances to be used in food contact materials, contaminants, non-authorised, prohibited and undesirable substances).
Delegated Regulation (EU) 2022/2292 supplements the Official Controls Regulation regarding specific public health requirements for food-producing animals and animal products exported to the EU.
The package of Regulations in Table 3 replaces Directive 96/23/EC.
Commission Regulation (EU) No 37/2010 sets maximum residue limits (MRLs) of pharmacologically active substances in foodstuffs of animal origin.
Implementing Regulation (EU) 2021/808 complements Regulations (2022/1644 and 2022/1646. It lays down rules concerning methods of sampling and laboratory analyses for residues of pharmacologically active substances in live food-producing animals, their body parts and fluids, excrements, tissues, products of animal origin, animal by-products, feed and water. It also lays down rules for interpretation of analytical results.
Implementing Regulation (EU) 2021/405 lays down the lists of exporting countries (or regions) permitted to export animals and animal products for human consumption to the EU.
Resources
Online resources from the European Commission:
- Guidelines on EU requirements for entry of animals and products of animal origin: Control plans for residues of veterinary medicines, pesticides and contaminants
- Official controls on imported products
- Commission Notice on a guidance document on implementation of the requirements for multi-annual national control plans
Sources
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.