Refusal of health claims on beta glucans, Affron, MegaNatural-BP and Frutalose
- Health claims
- Labelling
Summary
The European Commission has rejected health claims related to beta glucans and blood glucose; Affron® and anxiety; MegaNatural®-BP and blood pressure; and Frutalose® and bowel function.
European Commission rejects health claims for beta glucans and blood glucose; Affron® and anxiety; MegaNatural®-BP and blood pressure; and Frutalose® and bowel function
Commission Regulation (EU) 2023/1141 of 1 June 2023 refusing to authorise certain health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health
Update
The European Commission has rejected health claims related to beta glucans and blood glucose; Affron® and anxiety; MegaNatural®-BP and blood pressure; and Frutalose® and bowel function.
Impacted Products
health foods
What is changing?
The Commission has rejected four health claims on foods submitted by food business operators because they do not to comply with the conditions set out in Regulation (EC) No 1924/2006, and will not be authorised for use on foods.
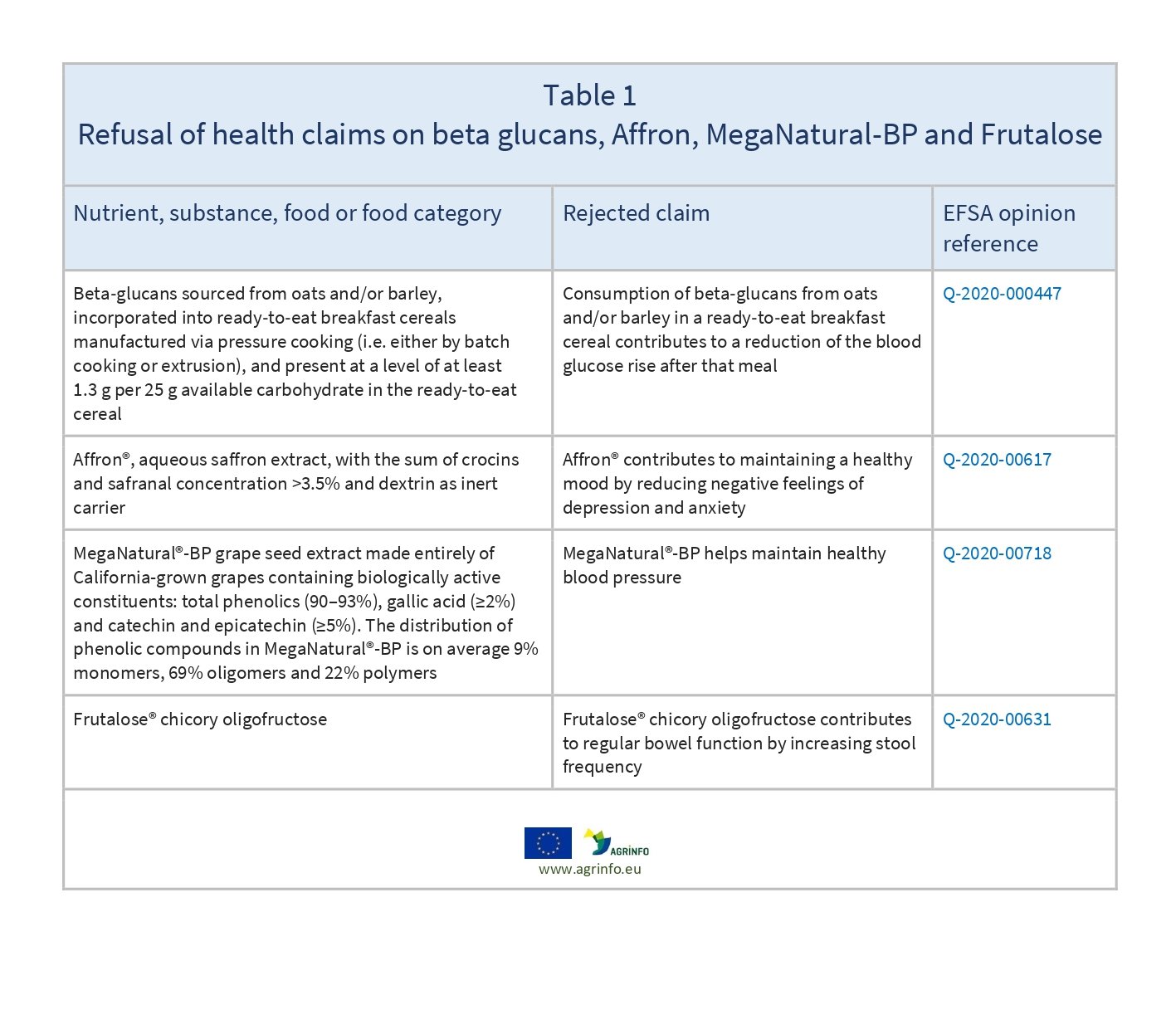
Table 1 sets out details of the four applications.
Why?
EFSA assessed all four health claims, with an unfavourable outcome. The evidence provided by the claimants was not sufficient to establish cause-and-effect relationships between consumption of the foods and the claimed health benefits.
Timeline
Entry into force: 2 July 2023.
What are the major implications for exporting countries?
The health claims listed in the Annex of the Regulation are not permitted on products exported to the EU.
Background
Health claims made on foods are prohibited unless they are authorised by the Commission in accordance with Regulation (EC) No 1924/2006 and included in the EU list of permitted claims. Food business operators may submit applications for authorisation of health claims to the national competent authority of a Member State. The national competent authority forwards applications to EFSA, which then delivers an opinion on the health claim concerned.
Resources
EFSA (2021) Beta-glucans from oats and/or barley in a ready-to-eat cereal manufactured via pressure cooking and reduction of blood-glucose rise after consumption: Evaluation of a health claim pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA Journal,19(4): 6493.
Sources
Commission Regulation (EU) 2023/1141 refusing to authorise certain health claims made on foods
Regulation (EC) No 1924/2006
Tables & Figures
Regulation (EU) 2023/1141
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
European Commission rejects health claims for beta glucans and blood glucose; Affron® and anxiety; MegaNatural®-BP and blood pressure; and Frutalose® and bowel function
Commission Regulation (EU) 2023/1141
What is changing and why?
The Commission has rejected four health claims on foods because they do not comply with the required conditions (Regulation (EC) No 1924/2006).
Health claims related to the following will not be authorised for use on foods:
- beta glucans and blood glucose
- Affron® and anxiety
- MegaNatural®-BP and blood pressure
- Frutalose® and bowel function.
Timeline
Proposed date of adoption: 2 July 2023.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
