List of non-EU country establishments – explained
- Animal health
- Food safety
- Animal health controls
- Food safety controls
- Official controls
Summary
Overview of the rules on listing food businesses (“establishments”) for the export of animals and animal products to the European Union (EU).
Rules for approval of establishments allowed to export animals and animal products to the EU
Commission Delegated Regulation (EU) 2022/2292 of 6 September 2022 supplementing Regulation (EU) 2017/625 with regard to requirements for the entry into the Union of consignments of food-producing animals and certain goods intended for human consumption
Commission Delegated Regulation (EU) 2020/692 of 30 January 2020 supplementing Regulation (EU) 2016/429 as regards rules for entry into the Union, and the movement and handling after entry of consignments of certain animals, germinal products and products of animal origin
Regulation (EU) 2017/625 of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products […] (Official Controls Regulation)
Regulation (EU) 2016/429 of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (Animal Health Law)
Update
Overview of the rules on listing food businesses (“establishments”) for the export of animals and animal products to the European Union (EU).
Background
To ensure that animal products are safe, the EU only imports these foods from approved operators in countries that are allowed to export animal products to the EU (see Third country lists for animal health – explained; Third country lists for public health – explained; EU official health certificates for exports to the EU – explained). Throughout the supply chain, these operators are known as “establishments” – that is, any unit of a food business (Regulation 852/2004, Art. 2(c)).
An establishment can only be approved if the competent authority in the non-EU exporting country has determined that it meets EU requirements, and has listed it on the TRACES Establishment Lists webpage.
Impacted Products
Animals, animal products, animal by-products, meat, fish, dairy, fats/oils, honey, sprouts
Overview
Types of establishment
For animal products imported to the EU from non-EU countries, establishments must be approved:
- where animals are kept after primary production (Regulation 2020/692, Art. 8)
- where animal products are prepared (Regulation 2022/2292, Art. 13)
- where products are dispatched from, if the establishment does more than just logistics, e.g. temperature control (Regulation 2022/2292, Art. 13)
- for fisheries: on-land establishments, factories, or freezer vessels, cold-stores or reefer vessels (Regulation 2022/2292, Arts. 18 and 19).
Once the approval procedure is completed, the competent authority assigns the operator a unique approval number.
Products
Establishments must be approved if they are exporting the following agri-food products (Harmonised System, HS codes):
- Animal products under HS chapters meat (2), fish (3), dairy (4), other animal origin (5), fats/oils (15), prepared meat/fish (16).
- Animal products under specific headings:
- other sugars (1702)
- chocolate (1806)
- coffee/tea extracts (2101)
- ice cream (2105)
- protein concentrates (2106)
- non-alcoholic beverages (2202 99)
- meat flours, meals (2301).
- Sprouts (0704 90, 0706 90, 0708 10, 0708 20, 0708 90, 1214 90) (see Regulation 2658/87).
- Honey (0409, 0410, 1212, 1521, 1702) (see Mandatory listing of establishments exporting honey, and clarifications on animal products).
Products containing substances of animal origin under the following Combined Nomenclature (CN) codes, often not destined for food use, must also only be exported by approved establishments:
- 2202 99, 2932, 3001, 3002, 3501, 3502, 3503, 3504, 3917 10 10, 4101, 4102, 4103.
For further details on which establishments must be approved in relation to each animal species, see Table 1.
Approval by the competent authority
To secure approval, before an establishment can start activities, the competent authority must control its compliance with the EU rules on-site.
This differs significantly from establishments supplying non-risk foods (products not of animal origin and shelf-stable composite products); or carrying out only primary activities (primary production including products of the soil, stock farming, hunting, and fishing, Regulation 852/2004); or transport or storage without temperature control – these establishments can begin activity as soon as they are registered, and only undergo controls at a later stage.
For live animals, detailed requirements to be fulfilled by the establishment are given in Regulation 2019/2035.
The approval number of the establishment must be indicated in the official health certificates accompanying exported food, and must be signed by the competent authority of the non-EU exporting country (see EU official health certificates for exports to the EU – explained).
Procedure for EU listing
Non-EU competent authorities can list approved establishments via the TRACES Establishment Lists webpage.
The European Commission will then check if all details are correct before publishing the list. Where mistakes are noted, the competent authority will be asked to review its whole list of establishments. National contact points in non-EU countries are responsible for updating the Establishment Lists.
See Non-EU countries authorised establishments.
What are the major implications for exporting countries?
The EU rules give significant powers to non-EU competent authorities to approve and control the establishments that are allowed to export to the EU. Every change in approved establishments must be communicated to the European Commission services.
Resources
Online resources from the European Commission:
- Guidance document on the implementation of certain provisions of Regulation (EC) No 853/2004 on the hygiene of food of animal origin
- IMSOC Establishment Lists
- Non-EU countries authorised establishments
PAFF Committee (2021) Technical Specifications for the Format for the Lists of Approved or Registered Establishments, Plants or Operators handling Animal By-Products inside the European Union and in Third Countries
AGRINFO Webinar and FAQ: Using TRACES
Sources
Commission Delegated Regulation (EU) 2022/2292 with regard to requirements for the entry into the Union of consignments of food-producing animals and certain goods intended for human consumption
Commission Delegated Regulation (EU) 2020/692 as regards rules for entry into the Union, and the movement and handling after entry of consignments of certain animals, germinal products and products of animal origin
Regulation (EU) 2017/625 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products […] (Official Controls Regulation)
Regulation (EU) 2016/429 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (Animal Health Law)
Tables & Figures
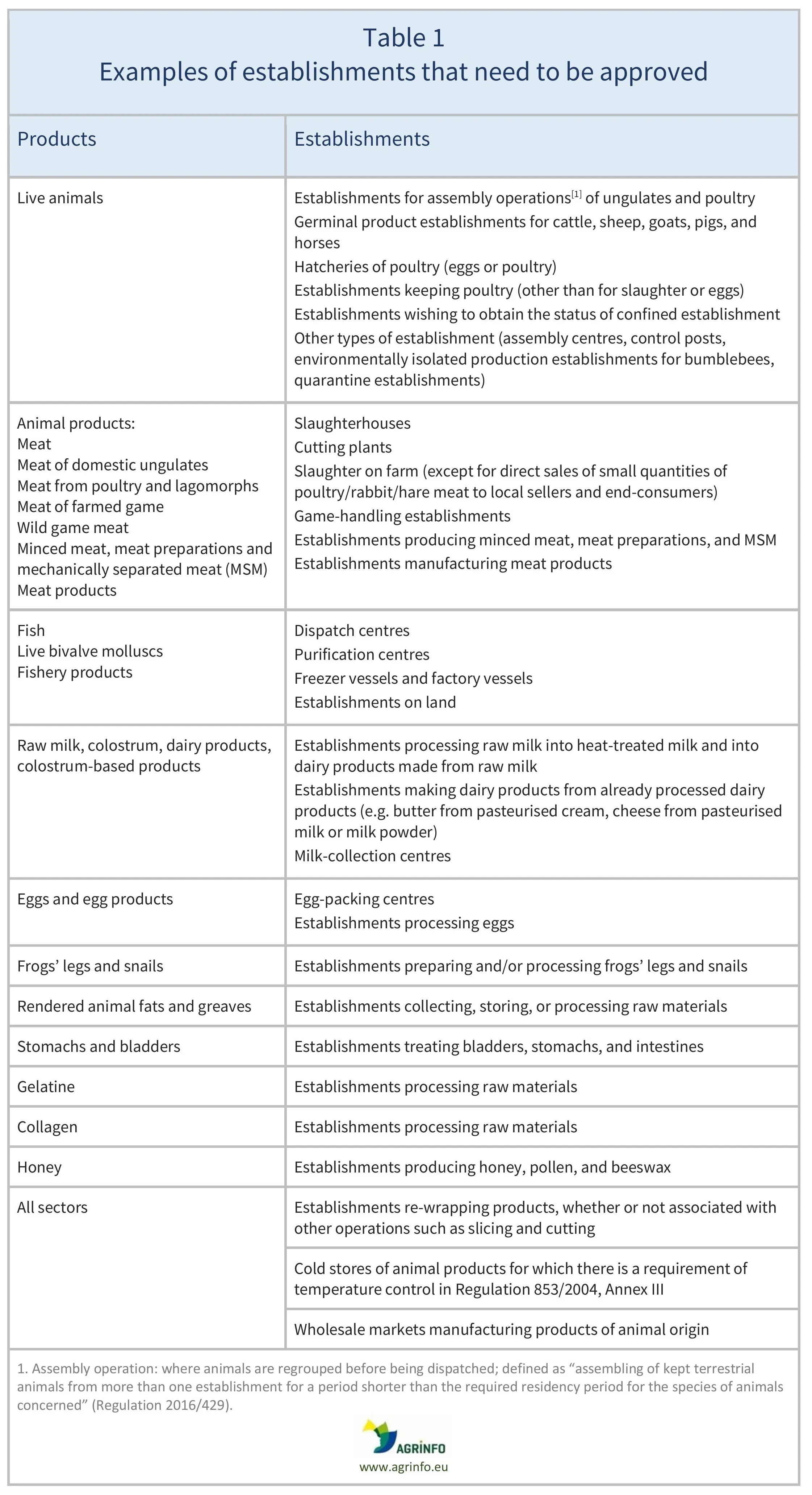
Sources: Live animals, Regulation 2016/429, Art. 94; Animal products, Guidance document on […] the hygiene of food of animal origin, and Regulation 2022/2292, Annex IV and Art. 13; Animal by-products, PAFF Committee (2021), Annex II, Ch. I.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
