EU Animal Health Law explained
- Animal health
- Animal health controls
- Official controls
Summary
An overview of the European Union (EU) Animal Health Law on transmissible animal diseases.
Overview of the EU Animal Health Law on transmissible animal diseases
Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (Animal Health Law)
Update
An overview of the European Union (EU) Animal Health Law on transmissible animal diseases.
Background
The Animal Health Law (Regulation 2016/429) is one of the major pieces of EU food legislation, along with the General Food Law (178/2002), the Official Controls Regulation (2017/625), and the Plant Health Law (2016/2031).
The EU has adopted a “One Health” approach, which considers that human, animal, and plant health are interlinked. It takes a common approach across sectors, placing an emphasis on disease prevention, including biosecurity, surveillance, and traceability.
Impacted Products
Animals (terrestrial and aquatic), germinal products (oocytes and semen), animal by-products, food of animal origin (meat, dairy/milk, eggs, certain fishery products)
Overview
The Animal Health Law deals with animal diseases that can be passed on to animals or to humans. It establishes principles and rules for the prevention and control of such diseases in animals kept by humans (including farm animals, fish, and aquaculture), wild animals, and animal products. The Regulation’s driving principle is that prevention is better than cure.
Scope and structure
The Animal Health Law applies to live animals (terrestrial, aquatic, wild), germinal products (oocytes and semen), animal by-products, and products of animal origin produced in the EU, and exported from non-EU countries to the EU. It covers the facilities where animals are reared and where products are produced, and the transport and equipment used that could play a role in spreading diseases.
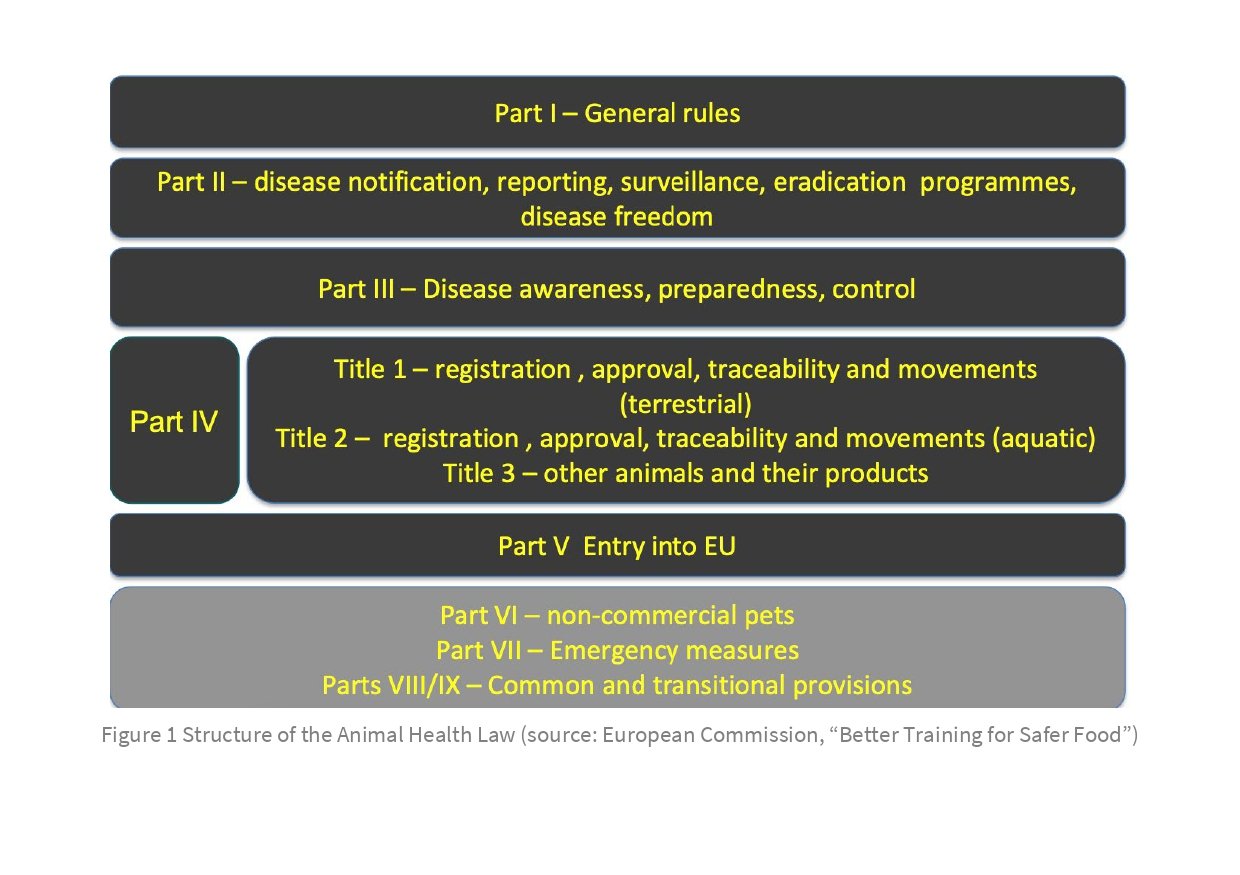
The basic logic and requirements of the Animal Health Law (Regulation 2016/429) are outlined in Parts I–IV; the specific requirements for non-EU countries exporting to the EU are set out in Part V (see Figure 1).
Basic requirements (Parts I–IV)
Parts I–IV of the Animal Health Law include the following.
- Definitions (Art. 2).
- Criteria to determine the animal diseases of concern for the EU, and the animal species subject to regulatory measures (Arts. 5.3 and 7).
- A new list of diseases relevant for EU intervention based on the above criteria (Art. 5, Annex II).
- Prioritisation and categorisation of diseases (Arts. 6–9).
- Requirements for competent authorities regarding early detection systems, notification and reporting of diseases, surveillance, eradication programmes, and disease-free status (Arts. 9 and 18–52).
- Basic responsibilities of animal keepers and veterinarians regarding health, biosecurity measures, early detection and prevention of animal diseases, surveillance (Art. 24), and animal health visits (Arts. 10–17 and 53–83).
- Obligation on operators for registration/approval by their competent authorities (Arts. 84–101; for aquaculture Arts. 172–185).
- Obligation on operators for notification of (possible) animal diseases to their competent authority (Art. 18) and subsequent actions by competent authorities (Arts. 19–23 and 53).
- Obligation on operators for record keeping (Arts. 102–107; for aquaculture Arts. 186–190).
- Traceability requirements for kept terrestrial animals and germinal products (Arts. 108–123).
- Obligations on farms for animal health visits by veterinarians (Arts. 25–27).
- Movements within the EU (Arts. 108–171 and 191–226).
- Animals and their products not covered by the definition of terrestrial and aquatic animals (Arts. 227–228).
Specific requirements for exports from non-EU countries to the EU (Part V)
To export to the EU (Art. 229), animals/animal products must:
- come from a non-EU country or territory that is approved and listed by the EU (this applies to the species/categories of animal products set out in Regulation 2021/404)
- come from an establishment producing animal products that is approved by the exporting country competent authority and listed by the EU
- fulfil all animal health requirements
- be accompanied by an animal health certificate and other relevant declarations/documents.
With the exception of certain low-risk products, all animal products entering the EU must be presented to an official border control post for checks.
Listing of countries
Countries can only be listed for the export of animals and animal products if they have control systems in place that are equivalent to EU requirements (Arts. 230–234). Before listing a country, the European Commission evaluates information provided relating to the animal health laws in place in the country of export, how controls are organised and carried out, certification procedures, and the country’s animal health status. If a disease outbreak occurs, or if there is information suggesting that a country no longer fulfils EU requirements, the Commission can remove a country or region from the list of authorised countries. This list of authorised countries can be found in Regulation 2021/404.
Approval of establishments
Animals and animal products (with the exception of composite products) must come from establishments that have been approved and are listed by the EU (Art. 233). It is the responsibility of the competent authorities in the exporting country to check that establishments comply with animal health requirements equivalent to EU measures. These Establishment Lists are publicly available within the EU’s Trade Control and Expert System (TRACES).
Animal health certificates and other documentation
Consignments of animals and animal products must generally be accompanied by an animal health certificate issued by the authority in the exporting country, as well as any other specific declarations or documents required (Art. 237). The certificates can be paper or electronic (through TRACES). Where possible it is recommended to use TRACES, as certificates are regularly updated and the most recent versions are automatically included in the system.
There are exceptions for certain types of product (e.g. shelf-stable composite products) which are considered to be a low risk for animal and public health. These products may require declarations or other documentation. There are also exceptions for products intended for personal use, and samples intended for use in research or to establish trade.
Certificates contain all the data on the products’ origin and destination, as well as information demonstrating that EU animal health requirements are met.
Other animal health rules relevant to non-EU countries
The Animal Health Law (Regulation 2016/429) provides the basic framework for animal health. There are detailed rules on:
- handling consignments entering the EU (Regulation 2020/692; see Animal health requirements for third countries exporting to the EU – explained)
- lists of non-EU countries authorised to export animals and animal products (Regulation 2021/404)
- risk categorisation of diseases by species (Regulation 2018/1882).
Timeline
Regulation 2016/429 applies from 21 April 2021.
An evaluation of the implementation and effectiveness of this Regulation is due by 22 April 2026.
What are the major implications for exporting countries?
Compliance with the EU animal health requirements is necessary for a non-EU country to be approved to export the following to the EU: animals (terrestrial and aquatic), germinal products, foods of animal origin (meat, dairy/milk, eggs, casings, certain fishery products), and processed animal ingredients in composite products. The specific requirements for non-EU countries exporting to the EU are described in Animal health requirements for third countries exporting to the EU – explained.
Resources
Online resources from the European Commission:
- About the Animal Health Law
- Better Training for Safer Food: New legislation on Animal Health [presentation]
- Animal health is your health [in 24 languages]
- Video: Animal Health Law [in 24 languages]
- List of delegated and implementing acts (as of 5 September 2022)
- Non-EU country establishments database
AGRINFO Webinar and FAQ: Using TRACES
Sources
Regulation (EU) 2016/429 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (Animal Health Law)
Tables & Figures
.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
