Animal health requirements for non-EU countries exporting to the EU – explained
- Animal health
- Food safety
- Animal health controls
- Food safety controls
- Official controls
Summary
Regulation 2020/692 supplements the European Union (EU) Animal Health Law with specific requirements for animals, germinal products, and animal products exported from non-EU countries to the EU.
Overview of Regulation 2020/692 on animal health requirements for non-EU countries exporting to the European Union
Delegated Regulation (EU) 2020/692 of 30 January 2020 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for entry into the Union, and the movement and handling after entry of consignments of certain animals, germinal products and products of animal origin
Update
Regulation 2020/692 supplements the European Union (EU) Animal Health Law with specific requirements for animals, germinal products, and animal products exported from non-EU countries to the EU.
Background
The EU has set rules regarding the production of any type of food. These include the General Food Law (Regulation 178/2002) and the Official Controls Regulation (2017/625).
For animal products, specific animal health requirements apply. These are set out in Regulation 2020/692, and supplement the Animal Health Law (Regulation 2016/429) – see EU Animal Health Law explained.
Impacted Products
Animals (terrestrial and aquatic), germinal products, food of animal origin (meat, dairy/milk, eggs, casings, certain fishery products), composite products
Overview
Regulation 2020/692 supplements the Animal Health Law (Regulation 2016/429) regarding the requirements for non-EU countries to be able to export animals and food of animal origin to the EU. It aims to prevent importing animal diseases that may be passed on to animals or humans.
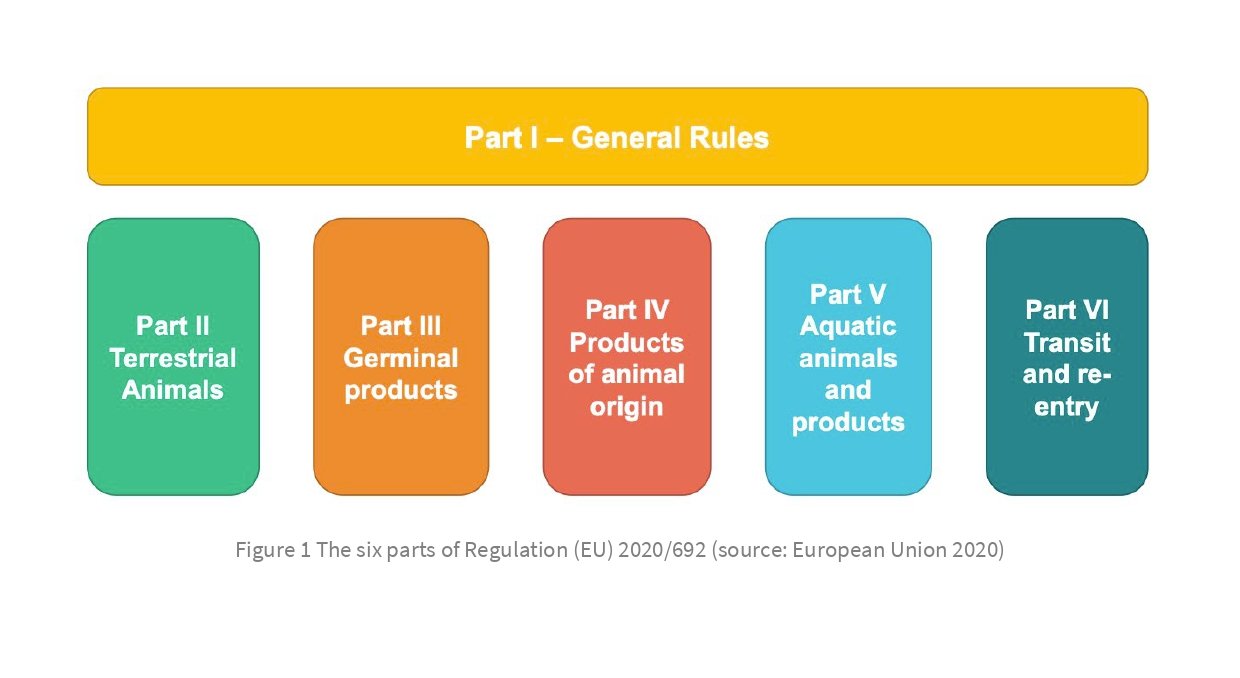
This Regulation is structured in six parts (see Figure 1).
I General rules
The general rules set out the scope, definitions, and general obligations.
Scope: the rules for exporting animals, germinal products, and foods of animal origin from non-EU countries to the EU, for meat (from all species), dairy/milk, eggs, and certain fishery products (Art. 1).
Obligations: the obligations for competent authorities (of both the non-EU country and the Member State), and for operators, are described (Arts. 3 and 5).
General animal health requirements
- Animals (exported live or used to produce animal products) must not show symptoms of transmissible disease, or come from areas where infections have been detected (e.g. a restricted zone or a country with a national programme for disease eradication) (Art. 7).
- Establishments must be registered or, for many animal products, approved (see Approval of third country establishments explained).
- Establishments must:
- keep up-to-date records on animals held in the establishment for a minimum of 3 years
- receive regular animal health visits from a veterinarian to assess animal health/disease status (Art. 8).
- When sampling and laboratory tests are required, samples must be taken by the competent authority and sent to an official laboratory (Art. 9).
- Animals intended for entry into the EU must come from a non-EU country or a zone that is free from category A diseases, and (depending on the species) free from category B and C diseases (Art. 10). Categories are detailed in the Annex to Regulation 2018/1882.
The EU does not grant disease-free status to non-EU countries, so countries wishing to export to the EU have to demonstrate freedom from disease in accordance with the EU legislation during a certain time period (Annex IV). For certain diseases, if the non-EU country or territory of origin is not completely free of the disease, risk-mitigating measures or conditions could be introduced.
II Specific requirements for kept terrestrial animals
Common rules
Minimum residency period
- Minimum residency periods for live kept terrestrial animals are given in Art. 11 (Annex III, tables 1 and 2); no animals are introduced into the establishment during the period indicated.
- Some derogations cold be introduced for registered horses for competitions, races, or cultural events (Art. 12).
Inspection
- Live terrestrial animals must be inspected by an official veterinarian in the non-EU country prior to export to the EU (Art. 13).
- Animals must be subject to a clinical inspection within 24 hours prior to the time of loading for dispatch to the EU.
- For poultry (except day-old chicks and captive birds), the inspection must also cover the flock of origin.
- For horses, the inspection may be carried out 48 hours prior to the time of loading or on the last working day.
Dispatch
- During the journey, the animals must not come into contact with other animals (Art. 14).
- Specific conditions are laid down for transhipment in non-listed non-EU countries (Arts. 15 and 16).
- There are specific transport requirements for animal safety and cleanliness (Arts. 17 and 18).
- Animals intended for slaughter must be slaughtered within 5 days from the date of arrival in the EU (Art. 19).
- In general, animals intended for raising must be kept for a minimum period of 30 days in the destination establishment (Arts. 19 and 26).
Specific rules
Ungulates
- In principle, animals must be delivered straight to their point of destination without passing through any other establishment. However, a single-assembly operation may be authorised under certain conditions (Art. 20).
- An obligation could be introduced for individual identification of ungulates intended for export to the EU: the code of the animal with a link to the animal health certificate and the ISO code of the exporting country (Art. 21).
- There are specific rules for entry of ungulates intended for confined establishments (Arts. 27–35).
Poultry, day-old chicks, and captive birds
Specific health requirements are laid down in Arts. 36–62. They concern in particular infections with highly pathogenic avian influenza and Newcastle disease virus.
Honeybees and bumblebees
- Only these two categories of bees can be exported to the EU.
- Specific health requirements are laid down in Arts. 63–72, in particular regarding packaging materials, cages, and feedstuffs.
III Germinal products
Requirements are set out as follows:
- germinal products of ungulates (Arts. 79–97)
- hatching eggs of poultry and captive birds (Arts. 98–116)
- other germinal products intended for confined establishments (Arts. 117–119).
The animal health requirements deal with the identification and residency period of donors/ flock of origin, the approval of establishments, traceability, transport, etc.
IV Products of animal origin
Requirements are set out as follows:
- general requirements (Arts. 120–123)
- fresh meat (Arts. 124–146)
- meat products and casings (Arts. 147–152)
- milk, dairy products, colostrum, colostrum products (Arts. 153–157)
- eggs and eggs products (Arts. 158–161)
- composite products (Arts. 162–165).
V Aquatic animals and their products
- General health requirements include: official inspection 72 hours prior to loading, rules of dispatch, transport conditions, labelling, freedom from disease (compulsory for categories A and B), registration or approval of establishments of origin, vector species, derogations, handling after entry into the EU (Arts. 166–174).
- Animal health requirements aim to limit the impacts of certain non-listed diseases (Art. 175).
VI Transit and re-entry
Requirements regarding transit through the EU, and products leaving the EU and returning to the EU, are given in Arts. 176–182.
VII Food for personal use
Some derogations to the animal health requirements could be introduced for some foods for personal use as far as they comply with certain conditions (maximum weight, packaging, etc.) (Arts. 164 and 165).
Annexes
I Notifiable diseases for germinal products, products of animal origin from ungulates, poultry and wild game, aquatic animals and their products (for terrestrial live animals, diseases are listed in Regulation 2016/429, Art. 5 and Annex II)
II Minimum information for disease surveillance programmes
III Minimum residency periods before exports to the EU for ungulates, honeybees, and bumblebees
IV Minimum periods of freedom from disease (Part A); conditions where the non-EU country is free from certain diseases for less than the period (Part B); and conditions in cases of vaccination or absence of vaccination (Part C)
V, VI Freedom from disease for tuberculosis, brucellosis, bluetongue, and leukosis
VI Additional requirements in cases of bovine rhinotracheitis, viral diarrhoea, and Aujeszky’s disease
VIII Restrictive areas to be applied for each disease in cases of outbreak
Timeline
Date of entry into application: 21 April 2021.
What are the major implications for exporting countries?
Non-EU countries must comply with the requirements laid down in this Regulation in order to be approved to export the following to the EU: live animals (and germinal products), meat [other than from horses, rabbits, Leporidae (wild rabbits, hares, etc.), and some other wild animals], milk/dairy, eggs, casings, and certain fish/fishery products.
Recommended Actions
For countries that are not yet listed, approval is a long process, but the EU market may be worthwhile for certain products. Competent authorities of AGRINFO partner countries may raise questions with SANTE-CONSULT-A5@ec.europa.eu.
For countries that are already listed, competent authorities and operators should ensure that they remain compliant. The EU regularly audits its partner countries and the EU Member States, and publishes Health and Food Audits and Analysis
Operators should be in regular contact with their competent authorities and support them in the process. Operators must also follow the procedure to be on the list of approved establishments (see Approval of third country establishments explained).
Resources
European Commission (2022) Animal Health Law: Key to the prevention and control of diseases in your animals [in 24 languages].
European Union (2020) Entry into the Union: New legislation on animal health [presentation].
Online resources from the European Commission:
- About the Animal Health Law
- Video: Animal Health Law [in 24 languages]
- Non-EU country establishments database
- Establishment Lists: Border Control Posts
Sources
Commission Delegated Regulation (EU) 2020/692 as regards rules for entry into the Union, and the movement and handling after entry of consignments of certain animals, germinal products and products of animal origin
Regulation (EU) 2016/429 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (Animal Health Law)
Tables & Figures
.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
