Exporting all parts of hemp plants to the EU
- Contaminants
- Food safety
Summary
The European Commission proposes to allow all hemp plant parts to be exported to the European Union (EU) as agricultural products. It also proposes to extend the maximum levels of delta-9-tetrahydrocannabinol (Δ9-THC), currently authorised for hemp seeds exported to the EU, to include all hemp plant parts (raw hemp, seeds, and other plant parts). Hemp seeds that are not for sowing do not have to comply with these requirements.
EU proposes to allow imports of all hemp plant parts as agricultural products, and to extend maximum Δ9-THC levels (currently for seeds) to all plant parts
Proposal for a Regulation of the European Parliament and of the Council amending Regulation (EU) No 1308/2013 as regards the school fruit, vegetables and milk scheme (‘EU school scheme’), sectoral interventions, the creation of a protein sector, requirements for hemp, the possibility for marketing standards for cheese, protein crops and meat, application of additional import duties, rules on the availability of supplies in time of emergencies and severe crisis and securities
Update
The European Commission proposes to allow all hemp plant parts to be exported to the European Union (EU) as agricultural products. It also proposes to extend the maximum levels of delta-9-tetrahydrocannabinol (Δ9-THC), currently authorised for hemp seeds exported to the EU, to include all hemp plant parts (raw hemp, seeds, and other plant parts). Hemp seeds that are not for sowing do not have to comply with these requirements.
Impacted Products
Raw hemp, hemp seeds, other hemp plant parts
What is changing?
The European Commission proposes to allow all hemp plant parts to be exported to the EU as agricultural products. It also proposes to extend the maximum levels of Δ9-THC, currently authorised for hemp seeds exported to the EU, to apply to all hemp plant parts (raw hemp, seeds, and other plant parts). This means that:
- levels of Δ9-THC must not exceed a 0.3% limit
- plants must be grown from certified hemp seed, or accompanied by a certificate stating that the Δ9-THC level of the hemp variety does not exceed 0.3%.
Hemp seeds that are not for sowing do not have to comply with these requirements.
See details in Table 1.
Why?
This change aims to align with the EU’s proposed new rules on hemp production and marketing in order to protect public health through seed certification and limiting levels of Δ9-THC. This will ensure compliance with international drug conventions.
It will also unify the rules across the EU. Currently, EU Member States have different rules regarding the use of whole hemp plants and its economic potential, despite the European Court of Justice ruling (ECJ 2020) that cannabidiol (CBD) from the whole Cannabis sativa plant is not a drug. Evidence from the European Food Safety Authority (EFSA 2020) suggests there is a low health risk from hemp varieties with ≤0.3% Δ9-THC.
Timeline
The proposed rules are likely to be adopted by late 2026 to early 2027.
Resources
ECJ (2020) Judgment of the Court (Fourth Chamber) of 19 November 2020. European Court of Justice.
EFSA (2020) Acute human exposure assessment to tetrahydrocannabinol (Δ9‐THC). EFSA Journal, 18(1): 5953.
Sources
Proposal for a Regulation as regards the school fruit, vegetables and milk scheme (‘EU school scheme’), sectoral interventions, the creation of a protein sector, requirements for hemp, the possibility for marketing standards for cheese, protein crops and meat, application of additional import duties, rules on the availability of supplies in time of emergencies and severe crisis and securities
Tables & Figures
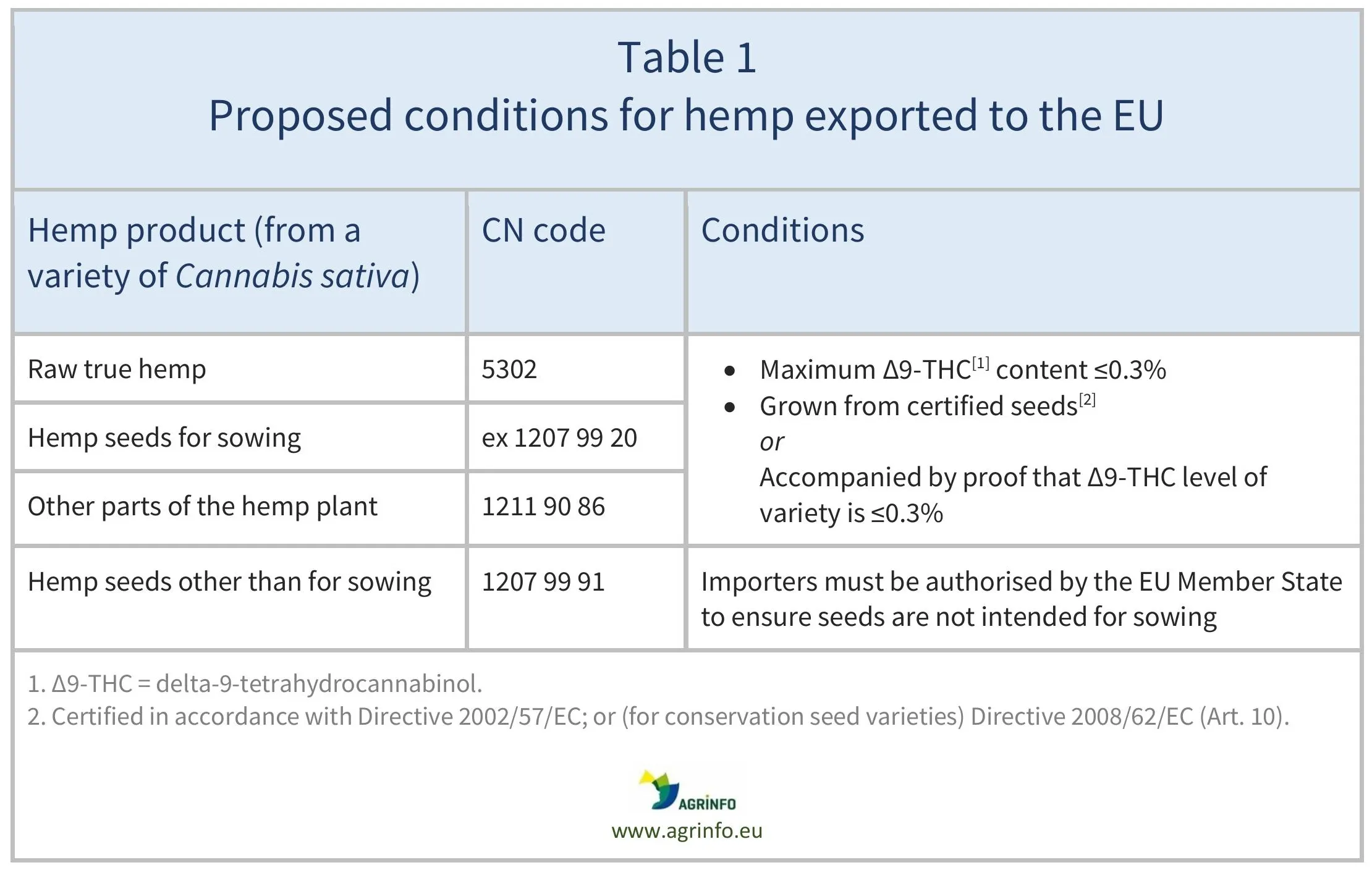
Source: based on Proposal amending Regulation 1308/2013
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU proposes to allow imports of all hemp plant parts as agricultural products, and to extend maximum Δ9-THC levels (currently for seeds) to all plant parts
Proposal for a Regulation as regards the school fruit, vegetables and milk scheme (‘EU school scheme’), sectoral interventions, the creation of a protein sector, requirements for hemp, the possibility for marketing standards for cheese, protein crops and meat, application of additional import duties, rules on the availability of supplies in time of emergencies and severe crisis and securities
What is changing and why?
The European Commission proposes to allow all hemp plant parts to be exported to the European Union (EU) as agricultural products. It also proposes to extend the maximum levels of delta-9-tetrahydrocannabinol (Δ9-THC), currently authorised for hemp seeds exported to the EU, to include all hemp plant parts (raw hemp, seeds, and other plant parts). This means that:
- levels of Δ9-THC must not exceed a 0.3% limit
- plants must be grown from certified hemp seed, or accompanied by a certificate stating that the Δ9-THC level of the hemp variety does not exceed 0.3%.
Hemp seeds that are not for sowing do not have to comply with these requirements.
See details in Table 1.
Timeline
The proposed rules are likely to be adopted by late 2026 to early 2027.
Tables & Figures

Source: based on Proposal amending Regulation 1308/2013
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
