Maximum levels of delta-9-tetrahydrocannabinol (Δ9-THC) in hemp seeds
- Contaminants
Summary
On 12 August 2022 the European Commission published a Regulation establishing maximum levels for delta-9-tetrahydrocannabinol (Δ9-THC) in hemp seeds and derived products.
European Commission publishes Regulation introducing maximum levels for delta-9-tetrahydrocannabinol (Δ9-THC) in hemp seeds and derived products
Commission Regulation (EU) 2022/1393 of 11 August 2022 amending Regulation (EC) No 1881/2006 as regards maximum levels of delta-9-tetrahydrocannabinol (Δ9-THC) in hemp seeds and products derived therefrom
Update
On 12 August 2022 the European Commission published a Regulation establishing maximum levels for delta-9-tetrahydrocannabinol (Δ9-THC) in hemp seeds and derived products.
Impacted Products
hemp seeds and derived products
What is changing?
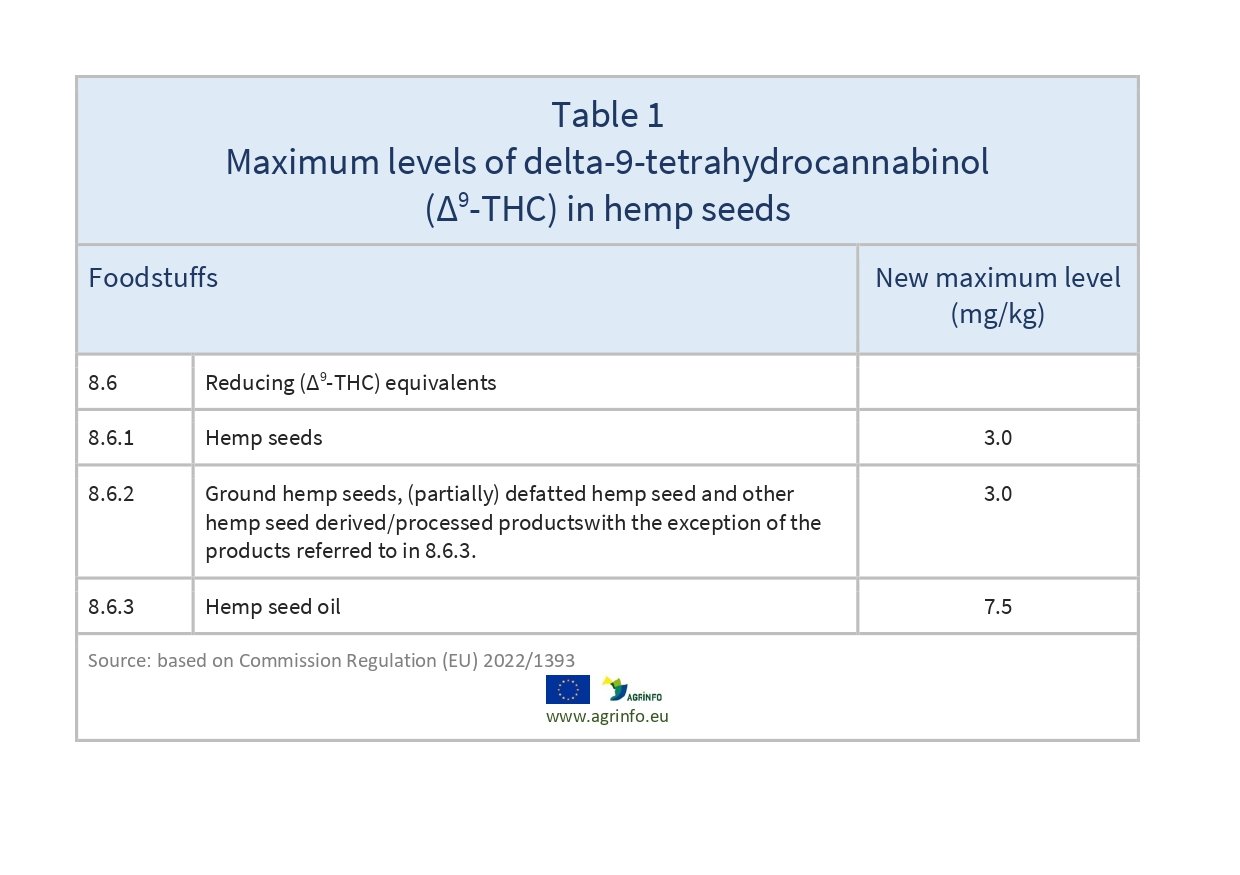
The new maximum levels are presented in Table 1.
Why?
In 2015, EFSA assessed the risk to human health related to the presence of Δ9-THC in milk and dairy products (EFSA 2015).
In 2020 EFSA published a scientific report assessing exposure to Δ9-THC, and found that the rate of 1 µg Δ9-THC per kg body weight as an acute reference dose was exceeded in certain exposure estimates (EFSA 2020). The Commission therefore considers that maximum levels should be set for hemp seeds and derived products.
Timeline
Date of publication: 12 August 2022.
Date of application: 1 January 2023.
Products lawfully placed on the market before 1 January 2023 may remain on the market until their date of minimum durability or use-by-date (even if they exceed the new maximum levels).
What are the major implications for exporting countries?
This regulation is of particular relevance to Chinese exporters, whose exports make up 94% of AGRINFO partner hemp seed exports to the EU. Lebanon and Paraguay are the next largest exporters.
Recommended Actions
Exporters of hemp seeds and derived products must ensure that adequate measures are put in place to prevent contamination during processing in order to meet the newly established levels (BfR 2018).
Resources
BfR (2018) Tetrahydrocannabinol levels are too high in many hemp-containing foods - health impairments are possible. Bundesinstitut für Risi.
EFSA (2015) Scientific Opinion on the risks for human health related to the presence of tetrahydrocannabinol (THC) in milk and other food of animal origin. EFSA Journal, 13(6): 4141.
EFSA (2020) Acute human exposure assessment to tetrahydrocannabinol (Δ9‐THC). EFSA Journal, 18(1): 5953.
Sources
Commission Regulation (EU) 2022/1393
Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006
Tables & Figures
.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
