French national MRL measures on carbendazim, thiophanate-methyl, glufosinate, and mancozeb
Summary
On 7 January 2025, the French Government issued an Order suspending the import and sale of some foodstuffs (raw or processed, including certain fruit, vegetables, soya beans, cereals, and honey) that originate from outside the European Union (EU) if they contain the following pesticides that are not approved for use in the EU: carbendazim (includes the sum of carbendazim and benomyl), thiophanate-methyl, glufosinate, and mancozeb.
This Order will apply from 8 February 2026. It applies only to foods put on the French market, and not to foods put on other EU Member State markets.
Individual EU Member States are permitted under EU law to take national emergency measures only where there is an evident serious risk to human health. The Order was notified to the World Trade Organization (WTO) on 9 January 2026.
On 20 January 2026, the European Commission and EU Member States discussed whether similar measures are required by the EU. The EU Member States did not support an EU-wide emergency measure, and will pursue the following actions that are already planned:
- carbendazim, thiophanate-methyl, and benomyl: publication of a draft Regulation lowering MRLs on all products
- glufosinate: update of risk assessment at EU level
- dithiocarbamates (including mancozeb): development of specific analytical methods.
The EU Member States did not support repealing the French Order, and it was agreed that France may maintain its own emergency measure (European Commission 2026).
France bans imports of certain foods containing residues of carbendazim, thiophanate-methyl, glufosinate, and mancozeb
Arrêté du 5 janvier 2026 portant suspension d’importation, d’introduction et de mise sur le marché à titre gratuit ou onéreux, en France, de denrées alimentaires provenant de pays tiers à l’Union européenne contenant des résidus de certaines substances actives phytopharmaceutiques interdites d’utilisation dans l’Union européenne
Update
On 7 January 2025, the French Government issued an Order suspending the import and sale of some foodstuffs (raw or processed, including certain fruit, vegetables, soya beans, cereals, and honey) that originate from outside the European Union (EU) if they contain the following pesticides that are not approved for use in the EU: carbendazim (includes the sum of carbendazim and benomyl), thiophanate-methyl, glufosinate, and mancozeb.
This Order will apply from 8 February 2026. It applies only to foods put on the French market, and not to foods put on other EU Member State markets.
Individual EU Member States are permitted under EU law to take national emergency measures only where there is an evident serious risk to human health. The Order was notified to the World Trade Organization (WTO) on 9 January 2026.
On 20 January 2026, the European Commission and EU Member States discussed whether similar measures are required by the EU. The EU Member States did not support an EU-wide emergency measure, and will pursue the following actions that are already planned:
- carbendazim, thiophanate-methyl, and benomyl: publication of a draft Regulation lowering MRLs on all products
- glufosinate: update of risk assessment at EU level
- dithiocarbamates (including mancozeb): development of specific analytical methods.
The EU Member States did not support repealing the French Order, and it was agreed that France may maintain its own emergency measure (European Commission 2026).
Impacted Products
Grapefruit, oranges, lemons, limes, clementines/tangerines, apples, pears, quinces, loquats, other pome fruits, apricots, cherries (sweet), peaches, plums, avocados, table grapes, wine grapes, mangoes, papayas, blackcurrants, strawberries, tomatoes, eggplant, okra, Brussels sprouts, beans (with pods), peas (with pods), cultivated mushrooms, potatoes, peppers, melons, lettuce, soya beans, barley, oats, rye, wheat, honey and other apiculture products
What is changing?
On 7 January 2025, the French Government issued an Order suspending the import and sale of some foodstuffs that originate from outside the EU if they contain certain pesticides that are not approved for use in the EU. The full decree in French can be seen here.
This measure by the French authorities addresses the following pesticides and their permitted residue levels on selected foods (raw or processed). The maximum residue levels (MRLs) will be set at the limit of quantification (LOQ) for the following substances: carbendazim (includes the sum of carbendazim and benomyl), thiophanate-methyl, glufosinate, and mancozeb. The foods affected are listed in Table 1. In France, importing these foods and placing them on the market will be prohibited if they contain residues of any of these pesticides. This measure only applies to the French market; the newly established French MRLs will therefore not be aligned with the EU MRLs currently in force across other EU Member States (see below).
This Order will enter into force on 8 January 2026, and there is a grace period of 1 month until it is applied. This very short transition period allows little time for producers and traders to adapt to these new requirements. The European Commission will discuss the French decision on 20 January. The Order was notified to the World Trade Organization (WTO) on 9 January 2026. Details on the pesticides concerned, and their current status within the EU, are as follows.
Carbendazim + benomyl and thiophanate methyl
Carbendazim (carbendazim + benomyl) and thiophanate-methyl are no longer authorised for use in the EU as no application was made by the manufacturers for their reapproval. When substances are not reapproved, MRLs are set to the LOQ, except on products for which an MRL based on uses outside the EU (an import tolerance) is considered safe in a risk assessment carried out by the European Food Safety Authority (EFSA). Import tolerances were in place for these two substances in citrus fruits, mangoes, papayas, and okra/lady’s fingers.
In 2024, following a review of these MRLs by EFSA that identified acute risks to consumer health, the European Commission proposed reducing these MRLs to the LOQ for some import tolerances:
- carbendazim in grapefruits, oranges, papayas, and mangoes
- thiophanate-methyl in grapefruits, oranges, mandarins, papayas, and mangoes.
For the other existing import tolerances for other foods, EFSA concluded that there is no risk for consumers and MRLs should remain, based on the good agricultural practices (GAP) from third countries:
- carbendazim in lemons, limes, mandarins, and okra/lady's fingers
- thiophanate-methyl in lemons, limes, and okra/lady’s fingers.
However, this 2024 proposal was rejected as a whole by the European Parliament because it objected to the setting of import tolerance MRLs for certain foods (see Maximum residue levels for benomyl, carbendazim, thiophanate-methyl, cyproconazole, and spirodiclofen). The European Parliament noted the public health risks associated with these substances, and also argued that allowing residues on imported foods for pesticides banned in the EU puts EU farmers at a competitive disadvantage. The Parliament’s objection prevented the European Commission from adopting this proposal, meaning that all the existing import tolerance MRLs continue to apply.
In February 2025, the Commission issued a new draft proposal to lower the MRLs to the LOQ on the products where EFSA identified acute consumer health risks (carbendazim and thiophanate-methyl). Adoption was originally planned for 2025, but is currently on hold pending ongoing discussions within the Commission. Due to the high risk, the new MRLs would apply 3 months after publication, rather than the usual 6 months.
Discussions on the remaining import tolerances, where EFSA (2021) did not identify risks for consumer health, are also ongoing.
Mancozeb
Mancozeb belongs to the dithiocarbamates group that also includes maneb, metiram, propineb, thiram, and ziram. In most cases MRLs are set for the group as a whole. While not approved for use in the EU, several import tolerance MRLs for the dithiocarbamates remain in place.
In 2024, the European Commission informed the WTO that it intends to amend the MRLs for dithiocarbamates in a wide range of products (G/SPS/N/EU/788). For details on the proposed changes and products affected, see Maximum residue levels for dithiocarbamates.
EFSA (2023) has reviewed the MRLs for dithiocarbamates. The analytical method used to quantify these substances is based on their conversion into carbon disulphide (CS2). CS2 can occur naturally in some plants, and EFSA used monitoring data from organic products to identify the natural CS2 content in certain plants, which is unrelated to (and should not be confused with) the use of pesticides.
The European Commission proposes setting the MRLs at the LOQ for products where the use of dithiocarbamates is not authorised in the EU, and if no import tolerances or Codex MRLs (CXLs) exist. For products where CXLs or import tolerances exist and are considered safe, the Commission proposes adjusting the MRLs accordingly.
As limited data is currently available for certain products, further evaluations and potential adjustments are still ongoing. Adoption of this proposal was originally planned for 2025 but is on hold pending further discussions within the Commission.
Against this background, there is a legal dispute around the European Commission's decision not to renew the approval of mancozeb. The decision was based on a European Chemicals Agency (ECHA) opinion, and has been challenged by EU producers who argue that it is based on flawed science and procedural errors, and is reliant on an opinion that is not legally binding. This dispute is ongoing.
Glufosinate
Glufosinate has not been approved for use in the EU since July 2018 as no application was made by the manufacturer for its reapproval. It is identified as a candidate for substitution.
Import tolerance MRLs currently remain in place for a number of products. Only one of these products is included in the French Decree: the MRL on potato will be reduced to the LOQ, while the EU import tolerance MRL applicable in the rest of the EU remains at 0.3 mg/kg for other foods (see the EU Pesticides Database).
Timeline
This Order will enter into force on 8 January 2026, and will apply from 8 February 2026.
Background
Under EU law, where an EU Member State informs the European Commission of an evident serious risk to human health, animal health, or the environment and the European Commission does not take measures to address that risk, a Member State may adopt interim protective measures (Regulation 178/2002, Arts. 53 and 54). This is the legal basis for the Order presented by France.
Resources
EFSA (2021) Reasoned opinion on the toxicological properties and maximum residue levels (MRLs) for the benzimidazole substances carbendazim and thiophanate-methyl. EFSA Journal, 19(7): e06773.
EFSA (2023) Review of the existing maximum residue levels for dithiocarbamates according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal, 21(5): e07987.
Regulation (EC) No 178/2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety.
European Commission (2026) Standing Committee on Plants, Animals, Food and Feed, Section Phytopharmaceuticals – Residues. Summary Report, 20 January.
Sources
Arrêté du 5 janvier 2026 portant suspension d’importation, d’introduction et de mise sur le marché à titre gratuit ou onéreux, en France, de denrées alimentaires provenant de pays tiers à l’Union européenne contenant des résidus de certaines substances actives phytopharmaceutiques interdites d’utilisation dans l’Union européenne
Tables & Figures
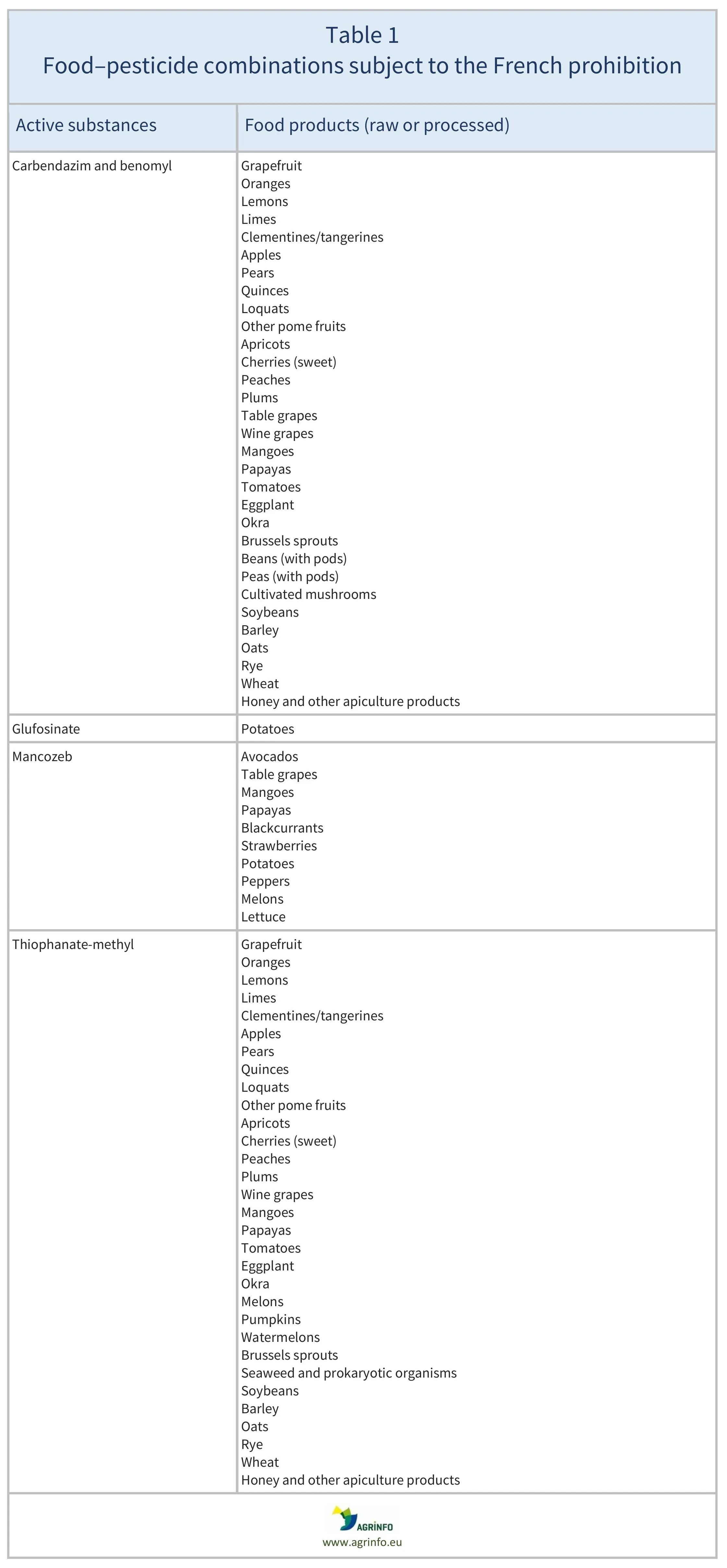
Source: based on Art. 1 of the French Order
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
France bans imports of certain foods containing residues of carbendazim, thiophanate-methyl, glufosinate, and mancozeb
Arrêté du 5 janvier 2026 portant suspension d’importation, d’introduction et de mise sur le marché à titre gratuit ou onéreux, en France, de denrées alimentaires provenant de pays tiers à l’Union européenne contenant des résidus de certaines substances actives phytopharmaceutiques interdites d’utilisation dans l’Union européenne
What is changing and why?
On 7 January 2025, the French Government issued an Order suspending the import and sale of some foodstuffs (raw or processed, including certain fruit, vegetables, soya beans, cereals, and honey) that originate from outside the European Union (EU) if they contain the following pesticides that are not approved for use in the EU: carbendazim (includes the sum of carbendazim and benomyl), thiophanate-methyl, glufosinate, and mancozeb.
This Order will apply from 8 February 2026. It applies only to foods put on the French market and not to foods put on the market in other EU Member States.
Individual EU Member States are permitted under EU law to take national emergency measures only where there is an evident serious risk to human health. The Order was notified to the World Trade Organization on 9 January 2026.
On 20 January 2026 the European Commission and EU Member States discussed the French Order. The EU Member States did not support an EU-wide emergency measure similar to that proposed by France, and instead will pursue actions that are already planned on these substances. The EU Member States did not support repealing the French Order, and it was agreed that France may maintain its own emergency measure.
Timeline
This Order will enter into force on 8 January 2026, and will apply from 8 February 2026.
Tables & Figures

Source: based on Art. 1 of the French Order
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
