Lipid and magnesium requirements for total diet replacement
- Food supplements/dietetic foods
Summary
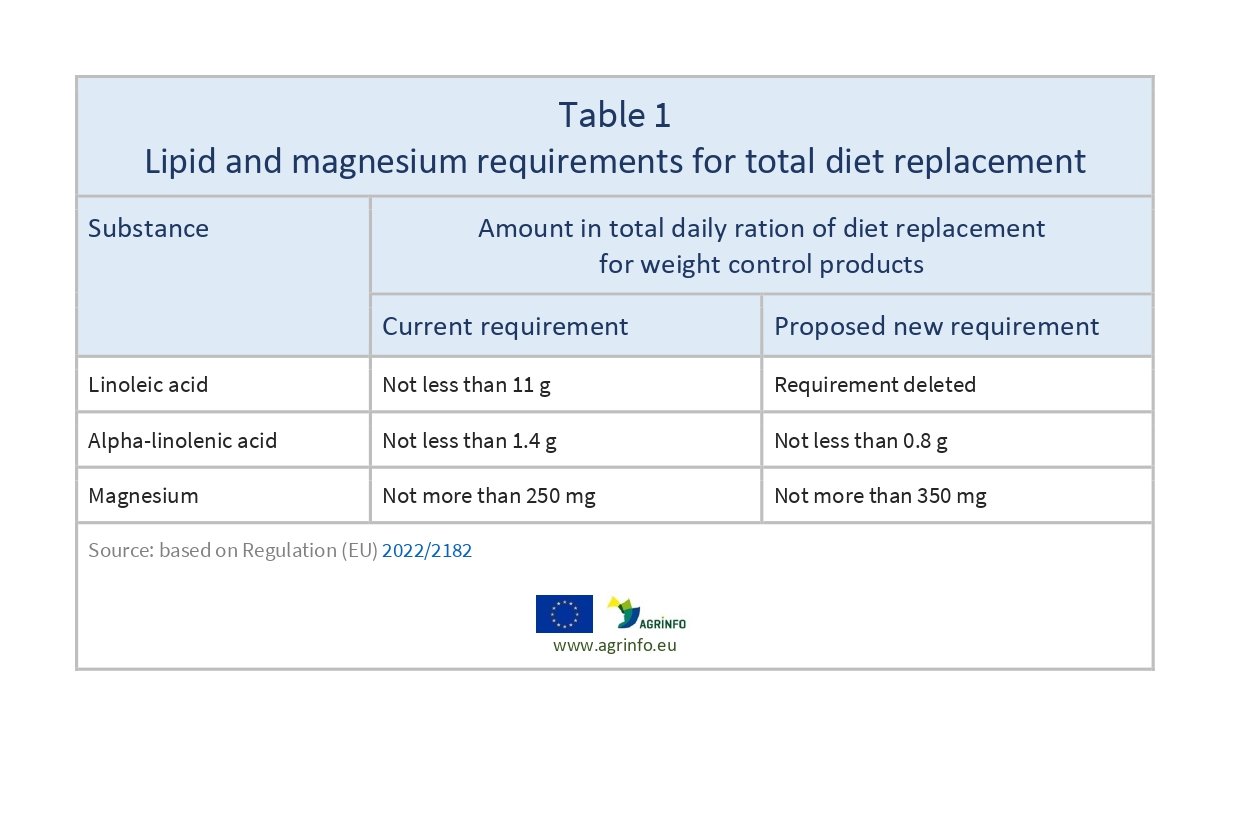
The European Commission has removed the linoleic acid requirement, lowered the alpha-linolenic acid requirement, and increased the magnesium content permitted in total diet replacements for weight control.
European Commission amends requirements for linoleic acid, alpha-linolenic acid, and magnesium in diet replacement products
Commission Delegated Regulation (EU) 2022/2182 of 30 August 2022 amending Delegated Regulation (EU) 2017/1798 as regards the lipid and magnesium requirements for total diet replacement for weight control
Update
The European Commission has removed the linoleic acid requirement, lowered the alpha-linolenic acid requirement, and increased the magnesium content permitted in total diet replacements for weight control.
Impacted Products
foods for special medical purposes
What is changing?
The Commission has changed the compositional requirements of linoleic acid, alpha-linolenic acid, and magnesium in total diet replacement for weight control products, as set out in Table 1.
Why?
The Commission received a stakeholder request, accompanied by new scientific evidence, to review current compositional requirements.
Timeline
Date of adoption: 30 August 2022
Background
Total diet replacement products for weight control are specially formulated for overweight or obese adults who want to reduce their weight. The EU has established rules to ensure that the daily nutritional requirements of overweight or obese adults in good health are met.
Delegated Regulation (EU) 2017/1798 specifies compositional requirements for these products, including requirements on energy values, and macronutrient and micronutrient content. Those requirements are based on the latest scientific advice from the European Food Safety Authority (EFSA).
Resources
EFSA (2021) Statement on additional scientific evidence in relation to the essential composition of total diet replacement for weight control. EFSA Journal, 19(4): 6494.
Sources
Tables & Figures
.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
European Commission amends requirements for linoleic acid, alpha-linolenic acid, and magnesium in diet replacement products
Regulation (EU) 2022/2182 amending Regulation (EU) 2017/1798 on lipid and magnesium requirements for total diet replacement for weight control
What is changing and why?
The European Commission has removed and lowered the requirements for linoleic acid and alpha-linolenic acid, and has increased the permitted magnesium content, in total diet replacement for weight control products. This is because the Commission received a stakeholder request, accompanied by new scientific evidence, to review these requirements.
Timeline
Date of adoption: 30 August 2022
Tables & Figures

.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
