Maximum residue levels for 1,4-dimethylnaphthalene
- Food safety
- Pesticide MRLs
- Pesticides
Summary
The European Commission has informed the World Trade Organization Sanitary and Phytosanitary Measures (WTO SPS) Committee (G/SPS/N/EU/899) that it intends to reduce the maximum residue levels (MRLs) for 1,4-dimethylnaphthalene from 0.05 to 0.03 mg/kg on all products of plant origin, except for potatoes.
EU discusses reducing MRLs for 1,4-dimethylnaphthalene on all plant products except potatoes
Draft Commission Regulation amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for 1,4-dimethylnaphthalene, chlormequat, metribuzin, metribuzin-desamino-diketo (metribuzin-DADK), terbuthylazine and triclopyr in or on certain products.
Update
The European Commission has informed the World Trade Organization Sanitary and Phytosanitary Measures (WTO SPS) Committee (G/SPS/N/EU/899) that it intends to reduce the maximum residue levels (MRLs) for 1,4-dimethylnaphthalene from 0.05 to 0.03 mg/kg on all products of plant origin, except for potatoes.
Impacted Products
Grapefruits, oranges, lemons, limes, mandarins, almonds, Brazil nuts, cashew nuts, chestnuts, coconuts, hazelnuts, macadamias, pecans, pine nut kernels, pistachios, walnuts, apples, pears, quinces, medlars, loquats/Japanese medlars, apricots, cherries (sweet), peaches, plums, table grapes, wine grapes, strawberries, blackberries, dewberries, raspberries (red and yellow), blueberries, cranberries, currants (black, red, white), gooseberries (green, red, yellow), rose hips, mulberries (black and white), azaroles/Mediterranean medlars, elderberries, dates, figs, kaki/Japanese persimmons, jambolans, kiwi fruits, litchis, passionfruits/maracujas, prickly pears, star apples/cainitos, American persimmons/Virginia kaki, avocados, bananas, mangoes, papayas, granate apples/pomegranates, cherimoyas, guavas, pineapples, breadfruits, soursops, table olives, kumquats, carambolas, durians, cassava roots, yams, arrowroots, sweet potatoes, beetroots, carrots, celeriac, horseradishes, Jerusalem artichokes, parsnips, Hamburg root parsley, radishes, salsifies, swedes/rutabagas, turnips, garlic, onions, shallots, spring onions/green onions, Welsh onions, tomatoes, sweet peppers/bell peppers, aubergines/eggplants, okra/lady’s fingers, gherkins, courgettes, cucumbers, melons, pumpkins, watermelons, sweetcorn, broccoli, cauliflowers, Brussels sprouts, head cabbages, Chinese cabbages/pe-tsai, kales, kohlrabies, lettuces, escaroles/broad-leaved endives, spinaches, purslanes, chards/beet leaves, witloofs/Belgian endives, lamb’s lettuces/corn salads, Roman rocket/rucola, red mustards, cresses and other sprouts and shoots, land cresses, watercresses, baby leaf crops (including Brassica species), chervil, celery leaves, parsley, sage, rosemary, thyme, basil and edible flowers, laurel/bay leaves, tarragon, grape leaves, beans and peas (with and without pods), lentils, asparagus, cardoons, celeries, Florence fennels, leeks, rhubarbs, bamboo shoots, palm hearts, globe artichokes, cultivated/wild fungi, mosses and lichens, algae and prokaryotes, beans, lentils, lupini beans, linseeds, peanuts/groundnuts, poppy seeds, sesame seeds, sunflower seeds, rapeseeds/canola seeds, mustard seeds, cotton seeds, safflower seeds, soyabeans, pumpkin seeds, castor beans, borage seeds, gold of pleasure seeds, hemp seeds, olives for oil production, oil palm kernels and fruits, kapok, barley, oat, buckwheat, maize/corn, millet, rice, rye, sorghum, wheat, chamomile, hibiscus, rose, jasmine, lime, strawberry, rooibos, maté, valerian, ginseng, aniseed, black cumin, celery, coriander, cumin, dill, fennel, fenugreek, nutmeg, cinnamon, cloves, capers, saffron, mace, allspice/pimento, Sichuan pepper, caraway, juniper berry, peppercorn (black, green, white), vanilla, tamarind, cardamom, liquorice, turmeric, sugar beet roots, sugar canes, chicory roots
What is changing?
The EU proposes to reduce MRLs for 1,4-dimethylnaphthalene on all products of plant origin, except for potatoes, from 0.05 to 0.03 mg/kg (Table 1).
MRLs for potatoes and animal products set in Regulation 2024/2640 (see Table 2) remain unchanged.
Why?
Recent monitoring data show that residues of 1,4-dimethylnaphthalene in products of plant origin can occur naturally at levels higher than the limit of determination (LOD, the lowest level that can be detected using the most modern and reliable analytical methods).
The MRL for 1,4-dimethylnaphthalene on potatoes is maintained at 20 mg/kg because the European Food Safety Authority has concluded that there is no consumer health risk for potato (EFSA 2023).
Timeline
The Regulation reducing the MRLs on plant products except potatoes is expected to be published in July 2026. It is expected that new MRLs will apply from late 2026 or early 2027.
Recommended Actions
Competent authorities of countries that are members of the WTO can submit comments on the EU’s proposal by emailing the EU SPS Enquiry Point until 1 February 2026.
Background
The setting of MRLs for potatoes and animal products (Regulation 2024/2640) followed assessment (EFSA 2023) of the impacts of livestock feed produced from potatoes in relation to 1,4-dimethylnaphthalene. It proposed lowering some existing MRLs in products from swine, cattle, sheep, goats, horses, and other farmed terrestrial animals, and in milk, but raising MRLs in poultry and egg products.
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
Commission Regulation (EU) 2024/2640 as regards maximum residue levels for 1,4-dimethylnaphthalene, difluoroacetic acid (DFA), fluopyram and flupyradifurone in or on certain products.
EFSA (2023) Modification of the existing maximum residue levels for 1,4-dimethylnaphthalene in potatoes. EFSA Journal, 21(8): art. 8190.
Sources
Draft Commission Regulation as regards maximum residue levels for 1,4-dimethylnaphthalene, chlormequat, metribuzin, metribuzin-desamino-diketo (metribuzin-DADK), terbuthylazine and triclopyr in or on certain products.
Tables & Figures
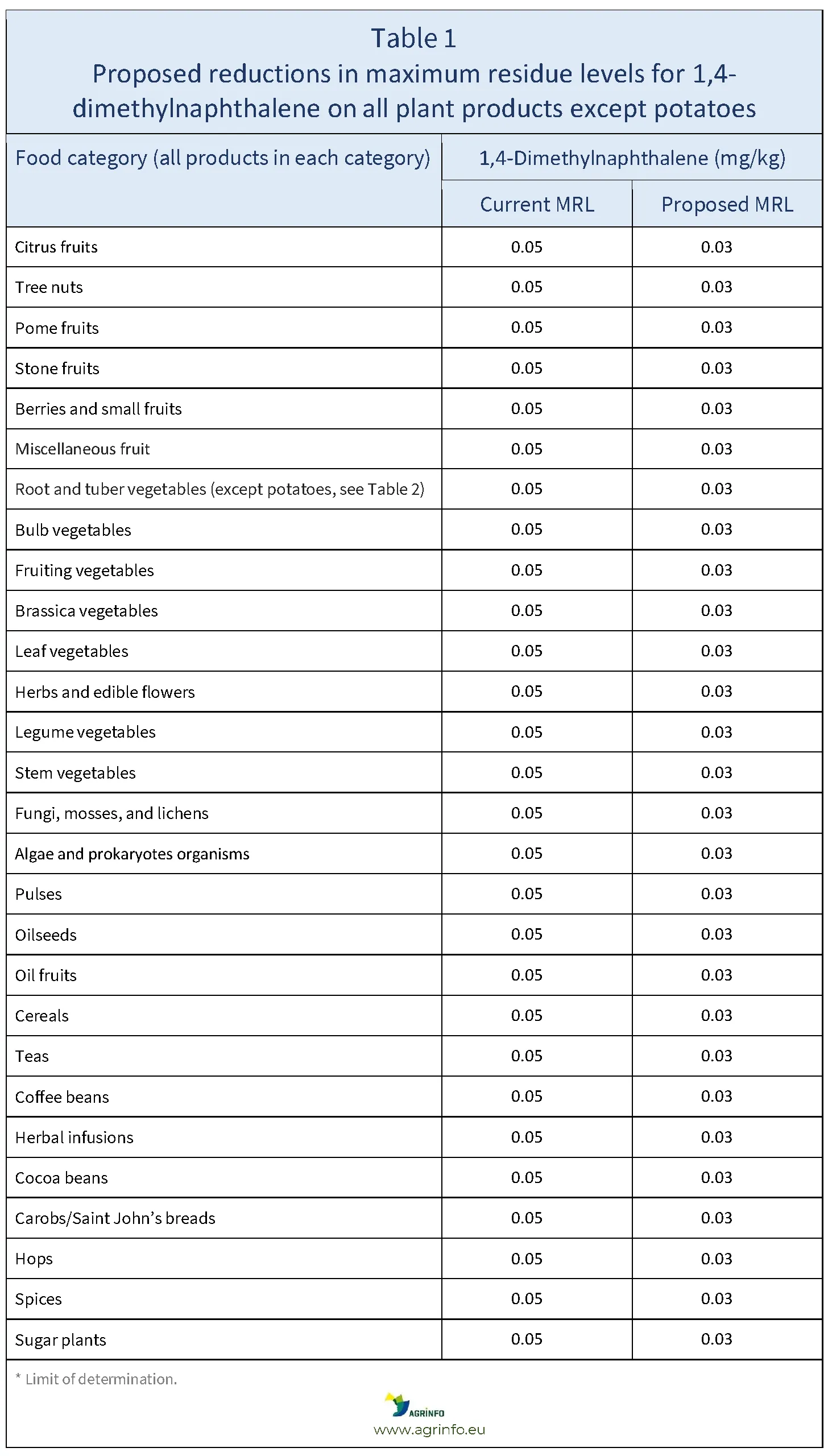
Source: based on PLAN-2025-1086_rev3 Draft Annex.
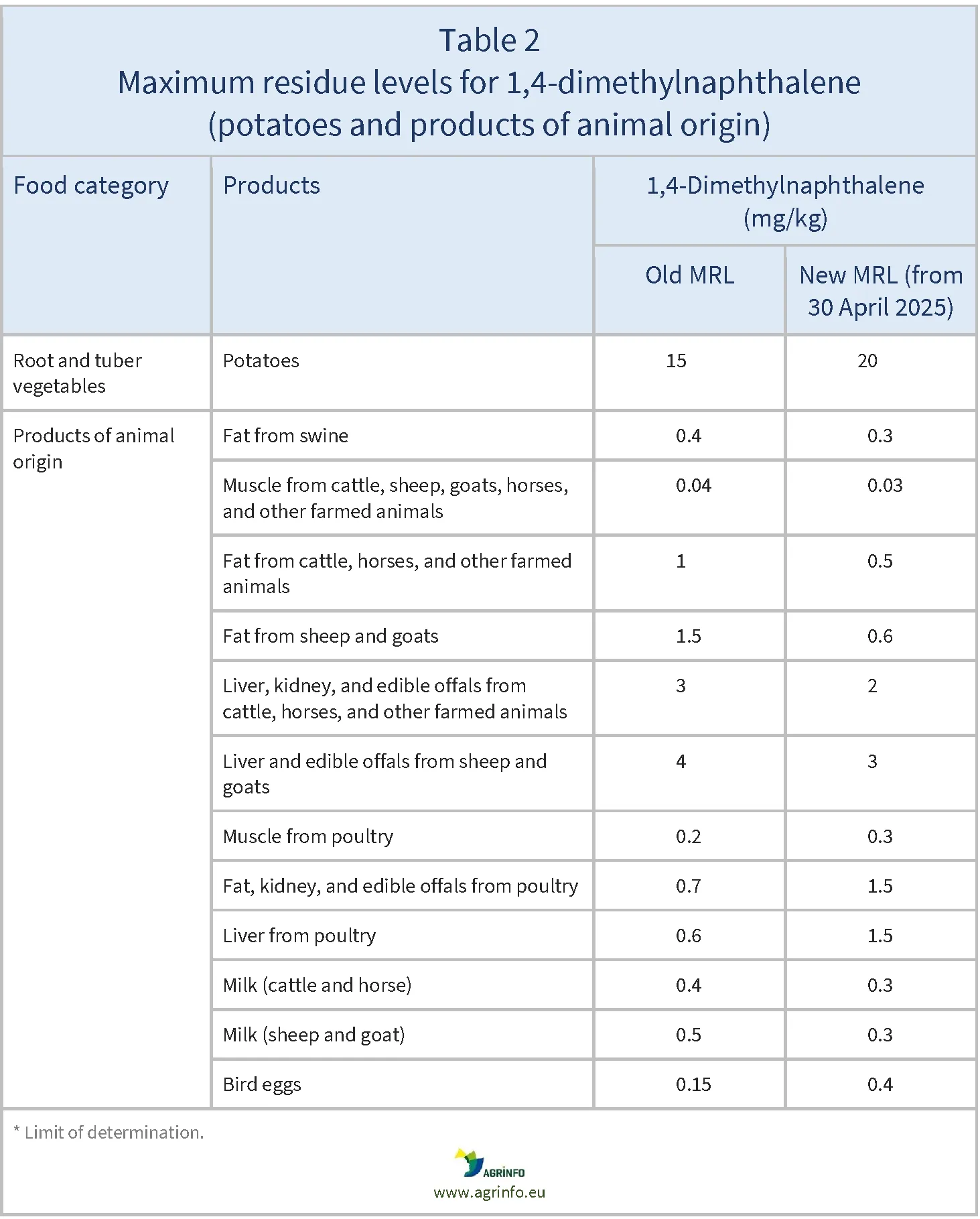
Source: based on Regulation (EU) 2024/2640.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU discusses reducing MRLs for 1,4-dimethylnaphthalene on all plant products except potatoes
Draft Commission Regulation as regards maximum residue levels for 1,4-dimethylnaphthalene, chlormequat, metribuzin, metribuzin-desamino-diketo (metribuzin-DADK), terbuthylazine and triclopyr in or on certain products.
What is changing and why?
The European Union (EU) proposes to reduce the maximum residue levels (MRLs) for 1,4-dimethylnaphthalene on all products of plant origin, except for potatoes, from 0.05 to 0.03 mg/kg (Table 1).
Recent monitoring data show that residues of this substance can occur naturally in foods of plant origin. Therefore, MRLs are set temporarily above the limit of determination (LOD, the lowest level that can be detected using the most modern and reliable analytical methods).
In October 2024, MRLs were set for potatoes and animal products as summarised in Table 2. These MRLs remain unchanged.
Actions
Competent authorities of countries that are members of the World Trade Organization can submit comments on the EU’s proposal by emailing the EU SPS Enquiry Point until 1 February 2026.
Timeline
The Regulation reducing MRLs in plant products is expected to be published in July 2026. It is expected that the new MRLs will apply from late 2026 or early 2027.
Tables & Figures

Source: based on PLAN-2025-1086_rev3 Draft Annex.

Source: based on Regulation (EU) 2024/2640.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
