Maximum residue levels for carbendazim
- Pesticide MRLs
- Pesticides
Summary
The European Union (EU) is discussing a new draft proposal to reduce the maximum residue levels (MRLs) for carbendazim to the limit of determination (LOD) on all products. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.)
This proposal follows the rejection by the European Parliament in September 2024 of a previous proposal by the European Commission to maintain the MRLs on lemons, limes, mandarins, and okra. See Maximum residue levels for benomyl, carbendazim, thiophanate-methyl, cyproconazole, and spirodiclofen.
EU discusses reduction of carbendazim MRLs to LOD on all products
Draft Commission Regulation amending Annex II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for benomyl, carbendazim and thiophanate-methyl in or on certain products
Draft Annex V [PLAN/2024/2763 v6]
Update
The European Union (EU) is discussing a new draft proposal to reduce the maximum residue levels (MRLs) for carbendazim to the limit of determination (LOD) on all products. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.)
This proposal follows the rejection by the European Parliament in September 2024 of a previous proposal by the European Commission to maintain the MRLs on lemons, limes, mandarins, and okra. See Maximum residue levels for benomyl, carbendazim, thiophanate-methyl, cyproconazole, and spirodiclofen.
Impacted Products
All products
What is changing?
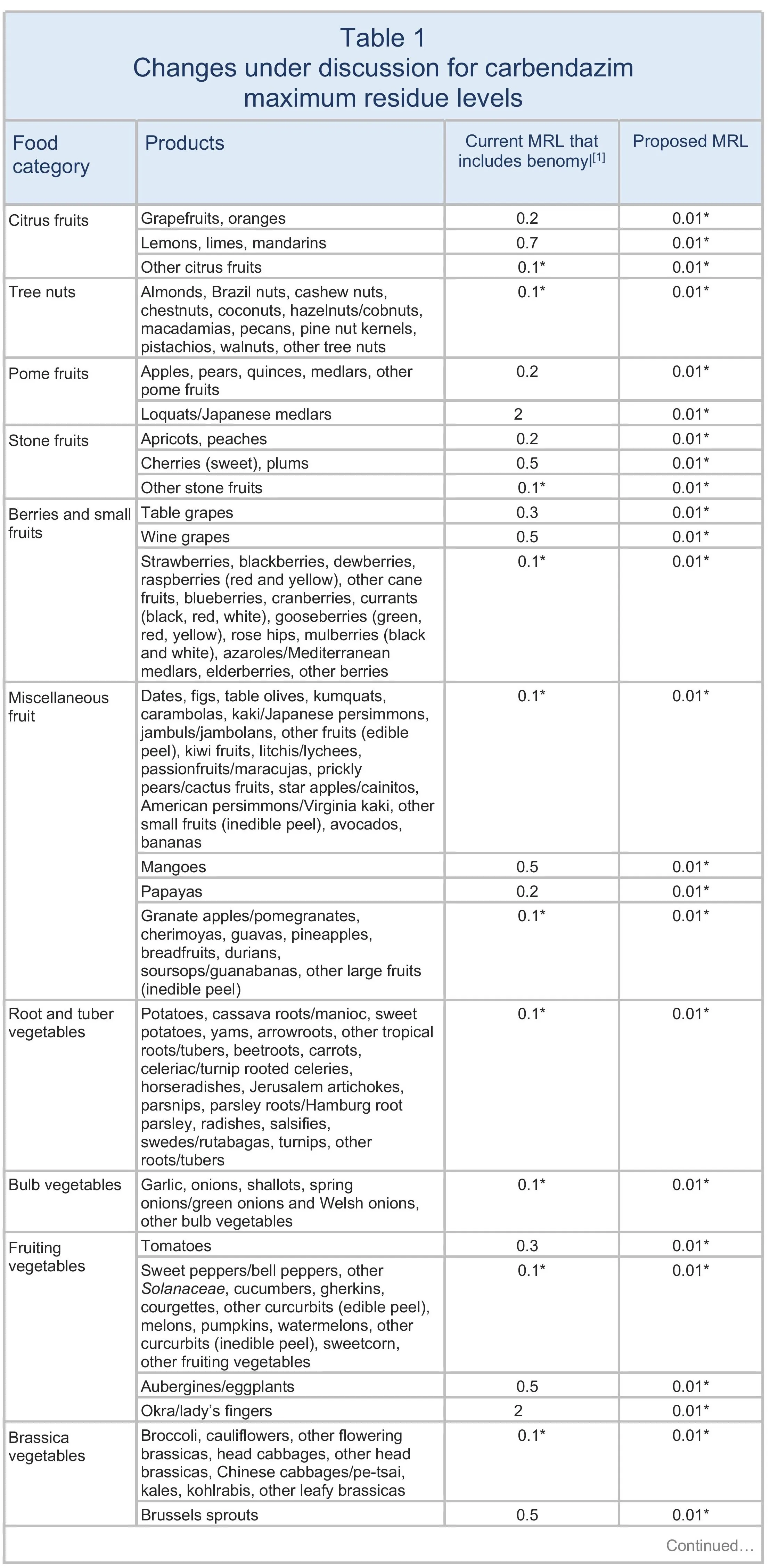
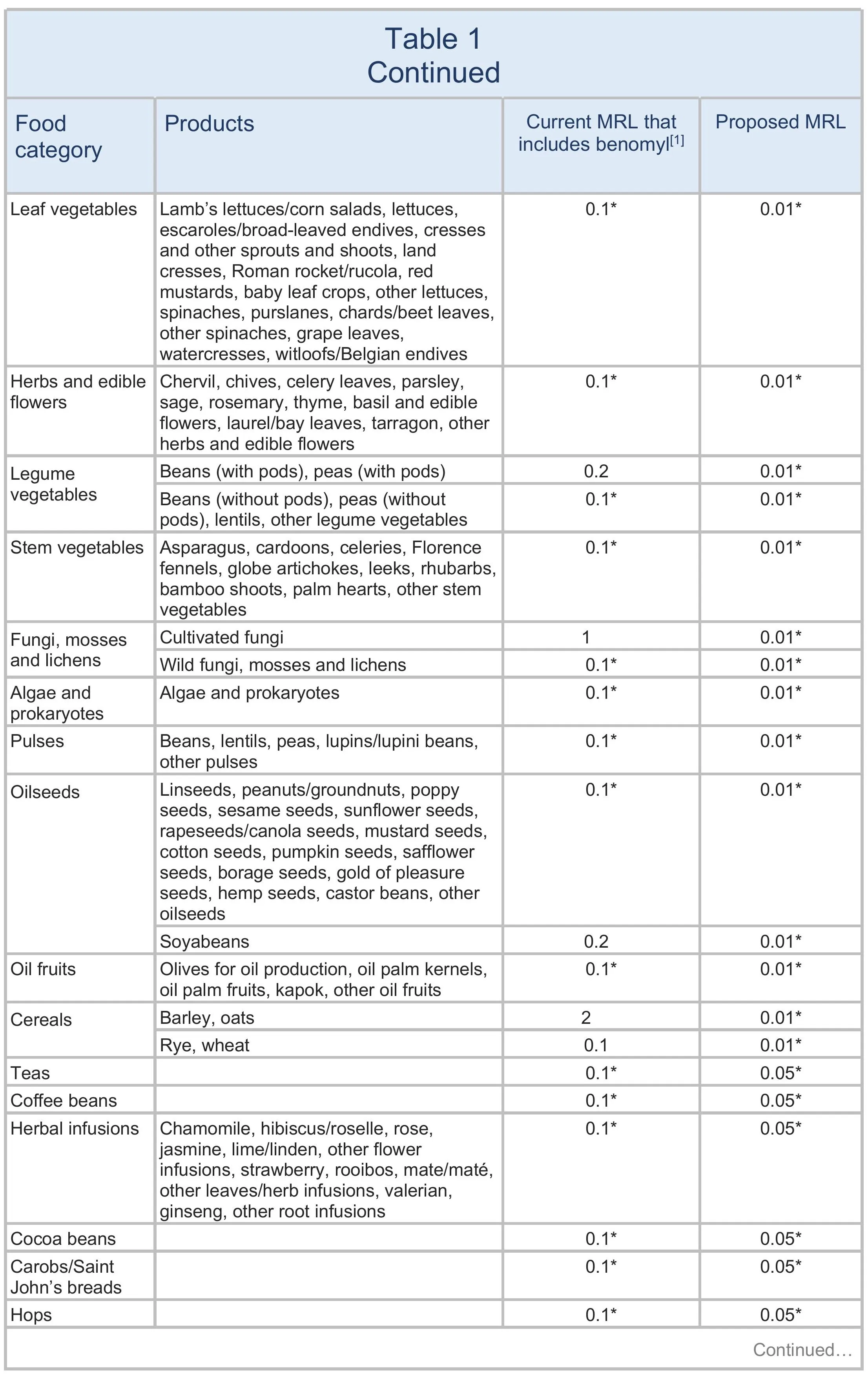
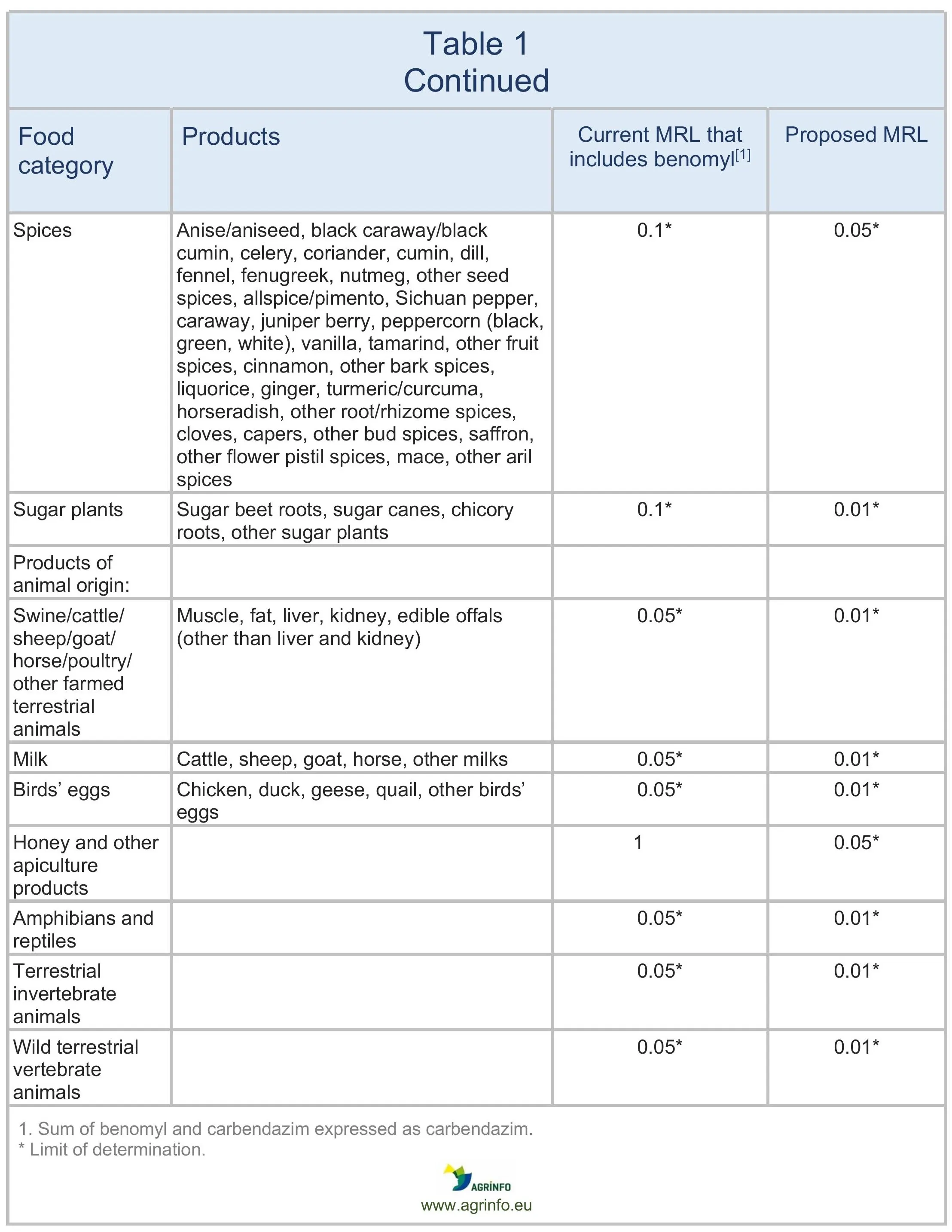
The European Commission has presented a draft proposal for discussion with EU Member States to lower the MRLs for carbendazim to 0.01 mg/kg on all products. The changes are summarised in Table 1. The current EU definition of carbendazim currently includes benomyl, but this proposal sets separate MRLs for the two substances. See Maximum residue levels for benomyl.
Why?
Carbendazim is no longer authorised in the EU as there has been no application for reapproval.
The European Food Standards Authority (EFSA 2021) suggested reducing the MRLs to the LOD, except on certain products that are considered safe. It proposed increasing the MRLs for carbendazim on lemons, limes, and mandarins, aligning with good agricultural practices (GAPs) observed in South Africa, and a lower MRL deemed safe for carbendazim on okra/lady’s fingers, also derived from GAPs in non-EU countries. However, the GAPs previously submitted are no longer authorised in South Africa. In addition, there are concerns about the risks of combined residues from carbendazim and thiophanate-methyl.
Timeline
The Regulation is expected to be published in 2026 and will apply 6 months after publication.
Recommended Actions
Suppliers to the EU market of all products should seek alternative chemical and non-chemical alternatives to the use of carbendazim.
Background
In September 2024, the European Parliament rejected a Commission Regulation that proposed to reduce the MRLs for carbendazim to the LOD on all products except lemons, limes, mandarins, and okra (see Maximum residue levels for benomyl, carbendazim, thiophanate-methyl, cyproconazole, and spirodiclofen). The Parliament requested the European Commission to withdraw its draft Regulation and present a new one, setting the MRLs for carbendazim on all products to the LOD.
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
EFSA (2021) Reasoned opinion on the toxicological properties and maximum residue levels (MRLs) for the benzimidazole substances carbendazim and thiophanate‐methyl. EFSA Journal, 19(8): 6773.
European Commission (2025a) Standing Committee on Plants, Animals, Food and Feed: Section Phytopharmaceuticals – Pesticide Residues. 17–18 February 2025. Summary Report.
European Commission (2025b) Standing Committee on Plants, Animals, Food and Feed: Section Phytopharmaceuticals – Pesticide Residues. 23–24 June 2025. Agenda.
Sources
Draft Commission Regulation as regards maximum residue levels for benomyl, carbendazim and thiophanate-methyl in or on certain products
Draft Annex V [PLAN/2024/2763 v6]
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU discusses reduction of carbendazim MRLs to LOD on all products
Draft Commission Regulation as regards maximum residue levels for benomyl, carbendazim and thiophanate-methyl in or on certain products
Draft Annex V [PLAN/2024/2763 v6]
What is changing and why?
The European Union (EU) is discussing a new draft proposal to reduce the maximum residue levels (MRLs) for carbendazim to the limit of determination (LOD) on all products. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.)
Actions
Suppliers to the EU market of all products should seek chemical and non-chemical alternatives to the use of carbendazim.
Timeline
The Regulation is expected to be published in 2026 and will apply 6 months after publication.
Tables & Figures
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.