Maximum residue levels for lambda-cyhalothrin
- Pesticide MRLs
Summary
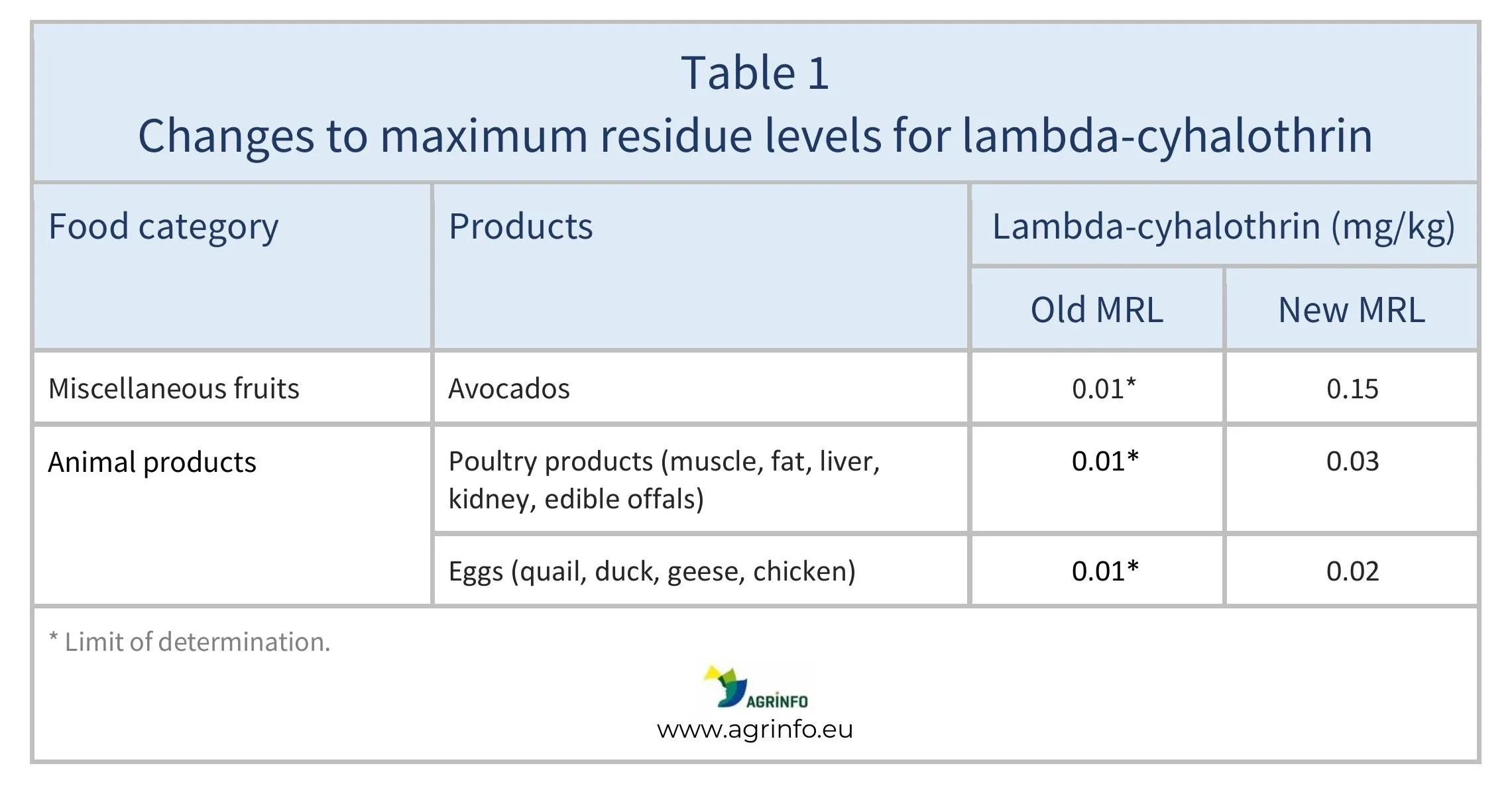
The European Union (EU) has increased the maximum residue level (MRL) for lambda-cyhalothrin on avocados following a request for an import tolerance. In addition, the EU has increased the MRL on poultry products and birds’ eggs, based on a dietary risk assessment concluding that the new levels proposed are unlikely to pose a risk to consumer health.
EU amends MRLs for lambda-cyhalothrin on avocados and poultry products
Commission Regulation (EU) 2025/115 of 21 January 2025 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for fluxapyroxad, lambda-cyhalothrin, metalaxyl, and nicotine in or on certain products
Update
The European Union (EU) has increased the maximum residue level (MRL) for lambda-cyhalothrin on avocados following a request for an import tolerance. In addition, the EU has increased the MRL on poultry products and birds’ eggs, based on a dietary risk assessment concluding that the new levels proposed are unlikely to pose a risk to consumer health.
Impacted Products
Avocados, poultry products (muscle, fat, liver, kidney, edible offals, eggs)
What is changing?
The EU has raised the MRLs for lambda-cyhalothrin as summarised in Table 1.
Why?
The EU received an application for an import tolerance MRL with reference to use of lambda-cyhalothrin on avocados in Mexico. On the basis of an evaluation by EFSA (2023), it was concluded that there are no risks to consumers at the proposed level.
In addition, MRLs for lambda-cyhalothrin on poultry products have been raised because an evaluation of monitoring data (EFSA 2024) found that residues exceeded the temporary MRLs set by Regulation 2018/960, due to authorised biocidal product use. EFSA therefore proposed new, higher MRLs that are unlikely to pose a risk to consumer health.
Timeline
The new MRLs apply from 11 February 2025.
Background
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
EFSA (2023) Setting of an import tolerance for lambda‐cyhalothrin in avocados. EFSA Journal, 21: e8464.
EFSA (2024) Targeted risk assessment of maximum residue levels for lambda-cyhalothrin in commodities from poultry and birds' eggs. EFSA Journal, 22: e8816.
Sources
Commission Regulation (EU) 2025/115 as regards maximum residue levels for fluxapyroxad, lambda-cyhalothrin, metalaxyl, and nicotine in or on certain products
Tables & Figures
Source: Based on Regulation 2025/115
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU amends MRLs for lambda-cyhalothrin on avocados and poultry products
Commission Regulation (EU) 2025/115 as regards maximum residue levels for fluxapyroxad, lambda-cyhalothrin, metalaxyl, and nicotine in or on certain products
What is changing and why?
The EU has increased the maximum residue levels (MRLs) for lambda-cyhalothrin on avocados and poultry products (see Table 1).
An import tolerance MRL for avocados from Mexico was approved after the European Food Safety Authority (EFSA) concluded that the proposed new level is not a risk to consumers.
MRLs for poultry products have also been raised after EFSA’s risk assessment found that higher residues due to authorised biocidal product use are unlikely to pose a risk to consumers.
Timeline
The new MRLs apply from 11 February 2025.
Tables & Figures

Source: Based on Regulation 2025/115
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
