Monacolin K from red yeast rice
- Food additives
Summary
The European Commission has introduced restrictions on maximum levels for daily consumption of the substance monacolin K from red yeast rice in food supplements.
European Commission establishes restrictions and warnings related to the use of monacolins from red yeast rice in food
Commission Regulation (EU) 2022/860 amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards monacolins from red yeast rice
Update
The European Commission has introduced restrictions on maximum levels for daily consumption of the substance monacolin K from red yeast rice in food supplements.
Impacted Products
food supplements
What is changing?
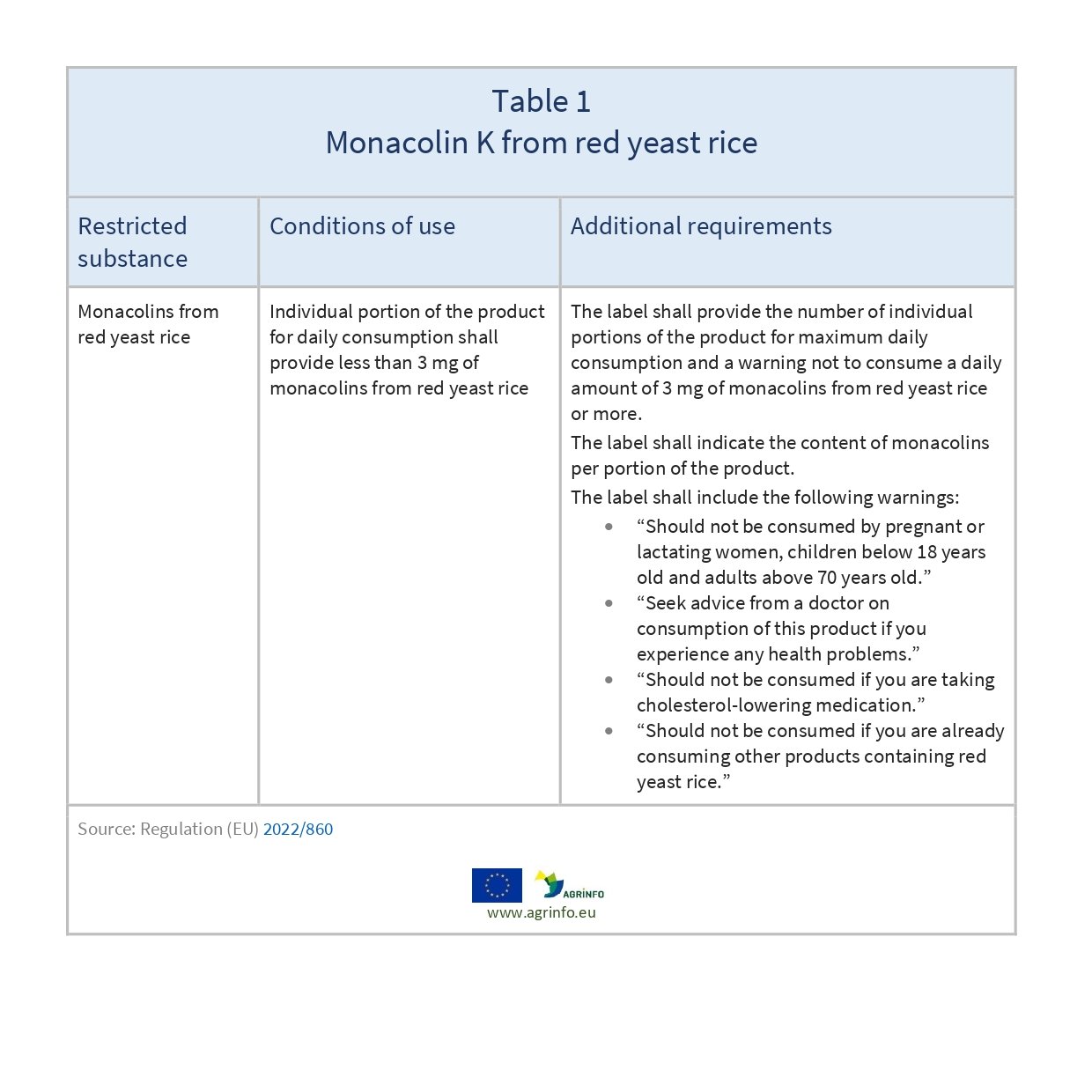
The European Commission is restricting maximum levels for daily consumption of the substance monacolin K from red yeast rice in food, amending Regulation (EC) No 1925/2006 (Annex III, Part B) as shown in Table 1.
As there is no scientific evidence to rule out risks to public health regarding the use of monacolins from red yeast rice, monacolins from red yeast rice is also added to the list of substances under Community scrutiny (Annex III, Part C).
Why?
Consumption of monacolins from red yeast rice is possibly associated with harmful effects on health, but there is scientific uncertainty. The substance is only used in food supplements, but the extent of that use could not be determined by EFSA. Therefore the use of this substance in foods, including food supplements, will be subject to continued regulatory scrutiny.
Timeline
Date of publication: 2 June 2022
Date of application: 22 June 2022
What are the major implications for exporting countries?
Red yeast rice is not authorised in the EU for use as a food colour, but it is marketed and consumed widely in food supplements (vitamins and dietary supplements). This use has come under increased EU scrutiny: monacolins from red yeast rice must now be limited in quantity and appropriate warning labels must be included.
AGRINFO partners exporting food supplements containing red yeast rice to the EU should clearly label the portion of the food supplement that is recommended for daily consumption, together with a warning not to exceed the stated recommended daily dose, as required by Directive 2002/46/EC (Art. 6). All foods containing red yeast rice must provide complete information about the monacolin content on the label.
Recommended Actions
In light of the scientific uncertainty and ongoing regulatory scrutiny, supplement producers using monacolins from red yeast rice should take the opportunity to submit data demonstrating their safety to EFSA within 4 years from this Regulation entering into force.
At that point the Commission should decide whether to list monacolins from red yeast rice in Part A “Prohibited substances” or Part B “Restricted substances”, in accordance with Regulation (EC) No 1925/2006, Art. 8(5).
Background
Red yeast rice is traditionally used in China as a food colouring, and as a remedy to promote digestion and blood circulation. Monacolins are produced during the manufacturing process of red yeast rice. The most abundant is monacolin K.
In the EU, red yeast rice is not authorised for use as a food colour, so it is not listed in Regulation (EC) No 1333/2008 on food additives.
Food supplements containing red yeast rice preparations are not subject to Regulation (EU) 2015/2283 on novel foods because they were already marketed and consumed widely before that regulation came into force. But the use of red yeast rice in other food categories is subject to authorisation under that Regulation.
Monacolin K (in lactone form) is identical to lovastatin, the active ingredient of medicinal products authorised in the EU for the treatment of high cholesterol (EFSA 2018). The intake of red yeast rice via food supplements could expose consumers to monacolin K at levels within the range of therapeutic doses of lovastatin. EFSA also noted that adverse effects to red yeast rice in humans are similar to those of lovastatin, affecting musculoskeletal and connective tissue (including rhabdomyolysis), liver, nervous system, gastro-intestinal tract, skin and subcutaneous tissue. These effects were judged to be of concern at the use level of 10 mg/day. Severe adverse reactions have been reported at intake levels as low as 3 mg/day taken for a period between 2 weeks and 1 year.
The composition and content of monacolins in food supplements containing red yeast rice, and in multi-ingredient products containing red yeast rice, have not been fully evaluated.
EFSA has received stakeholder comments arguing that adequate consumer information could support consumption of red yeast rice despite reports of associated side effects. Adverse effects are well documented for lovastatin, so harmful effects on health associated with the use of monacolins cannot be ruled out, and food supplements containing red yeast rice must be placed under EU scrutiny.
Resources
EFSA (2018) Scientific opinion on the safety of monacolins in red yeast rice. EFSA Journal, 16(8): 5368.
Sources
Tables & Figures
 .
.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
