Updated model animal health/official certificates for live terrestrial animals
- Animal health
- Animal health certification
Summary
The European Union (EU) has updated the animal health/official certificates that must accompany consignments of several categories of live animals (including cows, sheep, goats, pigs, poultry, and ratites), hatching eggs, semen, and embryos. The certificates have been updated to align with recent changes to rules on antimicrobial medicines, scrapie (transmissible spongiform encephalopathies, TSE) in goats, and the management of certain listed diseases.
EU updates animal health certificates for certain live terrestrial animals
Commission Implementing Regulation (EU) 2025/544 of 25 March 2025 amending Implementing Regulation (EU) 2021/403 as regards model animal health certificates and model animal health/official certificates for the entry into the Union of consignments of certain categories of terrestrial animals and germinal products thereof
Update
The European Union (EU) has updated the animal health/official certificates that must accompany consignments of several categories of live animals (including cows, sheep, goats, pigs, poultry, and ratites), hatching eggs, semen, and embryos. The certificates have been updated to align with recent changes to rules on antimicrobial medicines, scrapie (transmissible spongiform encephalopathies, TSE) in goats, and the management of certain listed diseases.
Impacted Products
Live animals, hatching eggs, embryos, semen
What is changing?
The EU has updated the model animal health certificates that must accompany consignments of the animals, hatching eggs, and germinal products (semen/embryos) listed in Table 1.
The updated model certificates can be found in the Annex to the new Regulation.
Why?
The model animal health/official certificates that must accompany consignments of live animals placed on the EU market have been updated taking into account the following.
- The attestation in the relevant certificates should include a reference to the list of non-EU countries and regions authorised as being compliant with rules on antimicrobial medicinal products (Regulation 2024/2598: see List of non-EU countries compliant with new EU antimicrobial requirements)
- For ovine and caprine animals (sheep and goats), the model animal health/official certificate (model ‘OV/CAP-X’ point II.2.12) should include a reference concerning caprine animals that are genetically resistant to classical scrapie strains (see TSE Regulation updated for goat strains resistant to scrapie)
- For animals (ruminants and horses) from confined establishments, references to infection with Brucella abortus, B. melitensis, B. suis, and Mycobacterium tuberculosis complex have been removed from the certificates.
- For certain categories of poultry, ratites (flightless birds), and their germinal products from certain zones, there is an additional alternative certification option regarding infection with Newcastle disease virus.
Timeline
The new models of the animal health certificates apply from 12 May 2025.
Until 12 November 2025, the previous models in use prior to this Regulation can still be issued. Consignments of animals accompanied by certificates issued before 12 November 2025 can be placed on the EU market until 12 February 2026.
Recommended Actions
It is important that exporters of live animals to the EU use the amended model certificates as soon as possible to ensure that all certificates are correct by 12 November 2025 and that no consignments will be rejected at the EU border.
Background
Countries exporting live animals and products of animal origin to the EU must send consignments together with animal health/official certificates signed by their official veterinarians. The aim is to guarantee that the consignments fulfil the EU legislation requirements.
These certificates are given in Regulation 2021/403 for terrestrial animals and germinal products (semen, ova, and embryos).
Resources
Commission Implementing Regulation (EU) 2021/403 laying down rules for the application of Regulations (EU) 2016/429 and (EU) 2017/625 as regards model animal health certificates and model animal health/official certificates, for the entry into the Union and movements between Member States of consignments of certain categories of terrestrial animals and germinal products thereof, official certification regarding such certificates and repealing Decision 2010/470/EU
Sources
Commission Implementing Regulation (EU) 2025/544 as regards model animal health certificates and model animal health/official certificates for the entry into the Union of consignments of certain categories of terrestrial animals and germinal products thereof
Tables & Figures
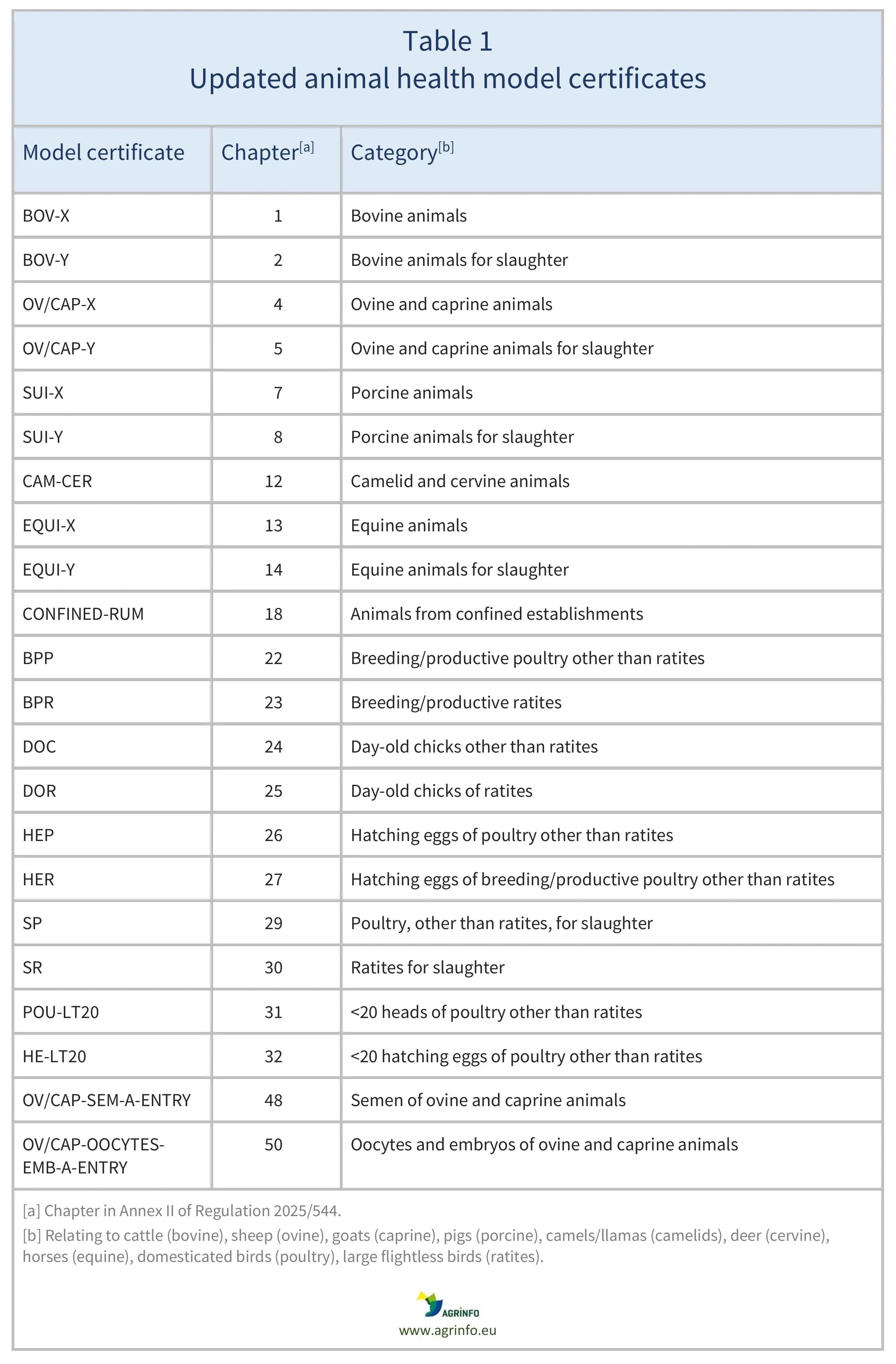
Source: Regulation 2025/544
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU updates animal health certificates for certain live terrestrial animals
Commission Implementing Regulation (EU) 2025/544 as regards model animal health certificates and model animal health/official certificates for the entry into the Union of consignments of certain categories of terrestrial animals and germinal products thereof
What is changing and why?
The European Union (EU) has updated the model animal health certificates that must accompany consignments of live animals (including cows, sheep, goats, pigs, poultry, and ratites [flightless birds]), hatching eggs, semen, and embryos. The updated certificates, listed in Table 1, are aligned with recent changes to rules on antimicrobial medicines, scrapie (transmissible spongiform encephalopathies, TSE) in goats, and the management of certain listed diseases.
Actions
It is important that exporters of live animals to the EU use the amended model certificates as soon as possible to ensure that all certificates are correct by 12 November 2025 and that no consignments will be rejected at the EU border.
Timeline
The new models of the animal health certificates apply from 12 May 2025.
Until 12 November 2025, the previous models in use prior to this Regulation can still be issued. Consignments of animals accompanied by certificates issued before 12 November 2025 can be placed on the EU market until 12 February 2026.
Tables & Figures

Source: Regulation 2025/544
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
