EU official health certificates for exports to the EU – explained
- Animal health certification
Summary
Overview of the rules relating to official certificates required for exporting animals and animal products to the European Union (EU).
Model health certificates for animals and animal products are laid down in three Commission Implementing Regulations:
Rules on official certificates required for exporting animals and animal products to the EU
Commission Implementing Regulations:
2021/403 as regards model animal health certificates and model animal health/official certificates, for the entry into the Union and movements between Member States of consignments of certain categories of terrestrial animals and germinal products thereof
2020/2235 as regards model animal health certificates, model official certificates and model animal health/official certificates, for the entry into the Union and movements within the Union of consignments of certain categories of animals and goods, official certification regarding such certificates
2020/2236 laying down rules for the application of Regulations (EU) 2016/429 and (EU) 2017/625 as regards model animal health certificates for the entry into the Union and movements within the Union of consignments of aquatic animals and of certain products of animal origin from aquatic animals, official certification regarding such certificates
Update
Overview of the rules relating to official certificates required for exporting animals and animal products to the European Union (EU).
Model health certificates for animals and animal products are laid down in three Commission Implementing Regulations:
- 2021/403 for live terrestrial animals and germinal products
- 2020/2235 for animal products, composite products, and live fish
- 2020/2236 for aquaculture (live fish).
Impacted Products
Live terrestrial animals and germinal products, animal products, composite products, aquaculture (live fish)
Overview
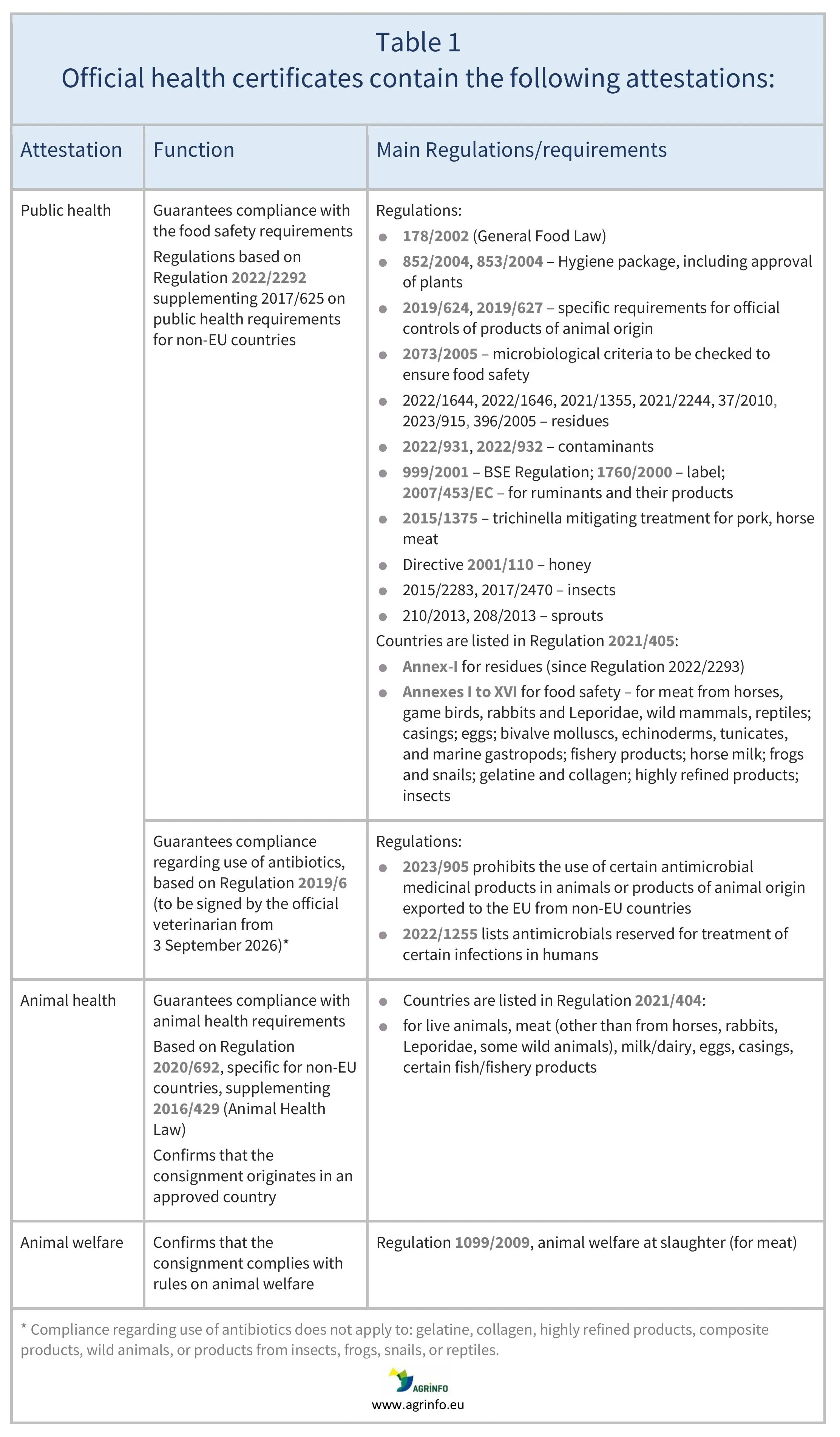
Official health certificates
Animals and animal products from non-EU countries can only enter the EU market if they are accompanied by an official certificate (except for shelf-stable composite products and highly refined products, where a private attestation is sufficient).
In addition to information identifying the product and its origin, the official certificate contains:
- a public health attestation
- an animal health attestation for animals (and germinal products), meat (other than from rabbits, Leporidae, and some wild animals), milk/dairy, eggs, casings, certain fish/fishery products)
- an animal welfare attestation (for meat).
See Table 1 for details on each attestation and the relevant Regulations.
Official certificates must be signed by the official veterinarians in non-EU countries. The certificates are guarantees that the consignment complies with EU legislation as set out in Table 1. Certificates are always checked during EU official controls at border control posts.
Certificates may be paper or electronic. The electronic form must be submitted via the EU system TRACES NT (see TRACES NT Documentation). The electronic form should be used wherever possible as it reduces the risk of mistakes (such as using an out-of-date certificate), and it is easier to correct.
A single mistake in the certificate is sufficient for a consignment to be rejected at the point of entry into the EU. It is crucial that the certificate is filled in carefully both by the operator (Part I) and by the non-EU country competent authority (Part II, also Part I if not filled by the operator).
One common mistake is to incorrectly state the weight of the goods. While this is not a sanitary issue, it will lead to rejection of the goods.
Issuing official certificates
Official certificates can only be issued in non-EU countries that are on the relevant lists for each species and category of animals in relation to:
- public health (see Third country lists for public health – explained)
- animal health (see Animal health requirements for third countries exporting to the EU – explained)
- antimicrobials (from 3 September 2026 – see List of non-EU countries compliant with new EU antimicrobial requirements).
Certificates can only be issued to individual establishments that are listed on the European Commission’s Establishment Lists webpage (see Approval of non-EU country establishments explained).
In the case of live animals and hatching eggs, the certificate must be issued within the 10 days prior to the consignment’s arrival. This period can be extended if consignments are travelling by sea (Regulation 2020/692, Art. 3c).
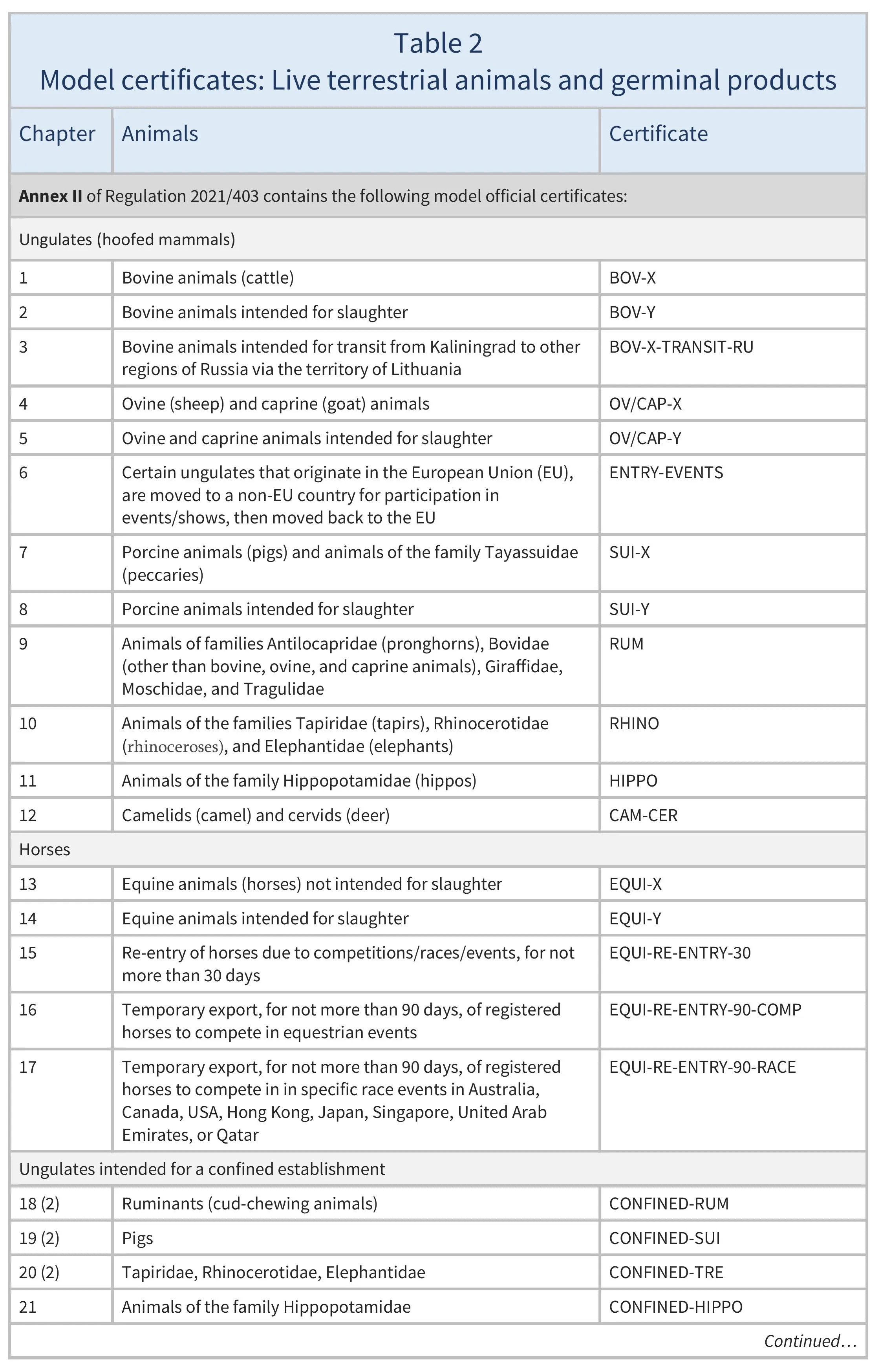
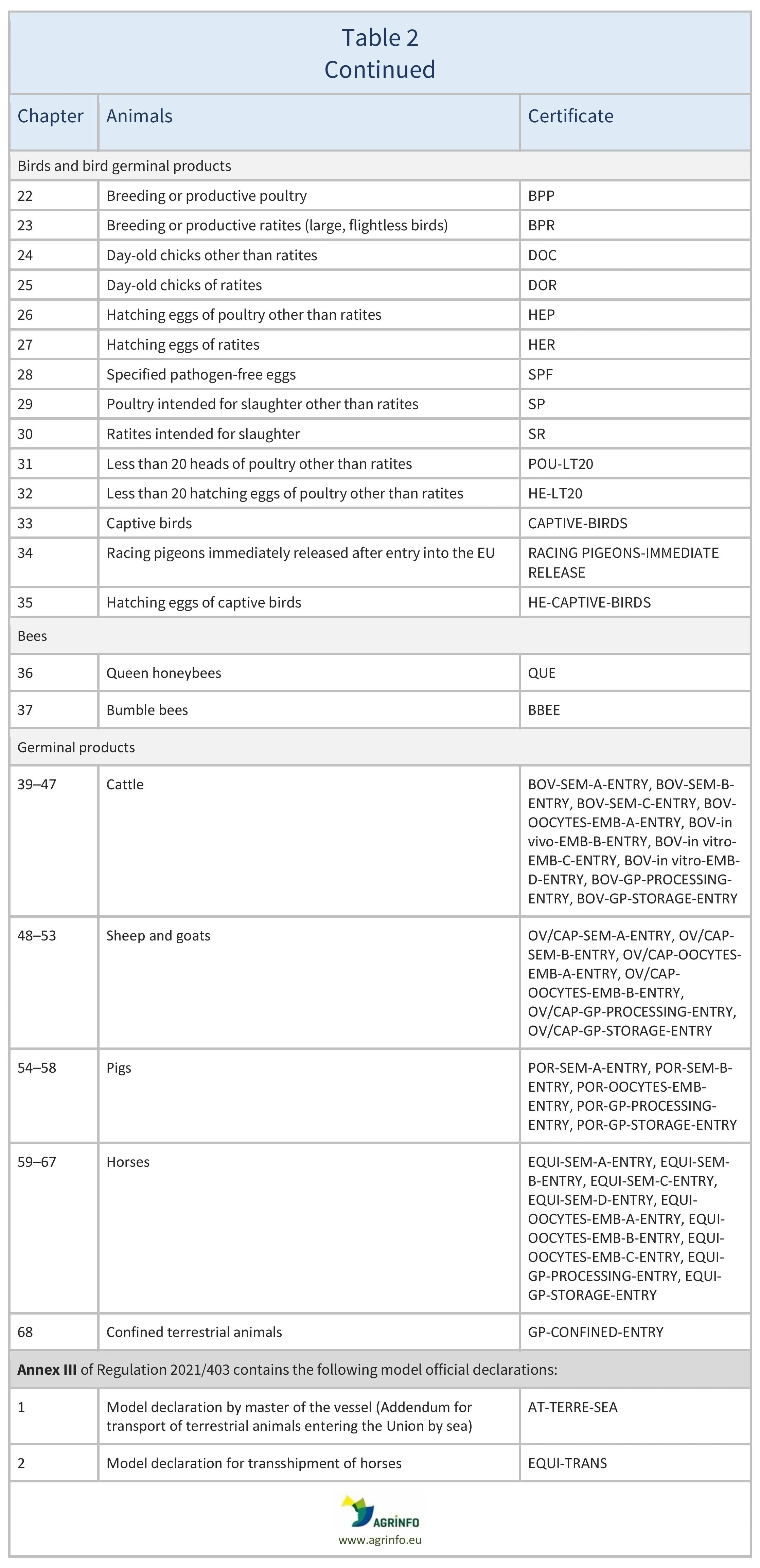
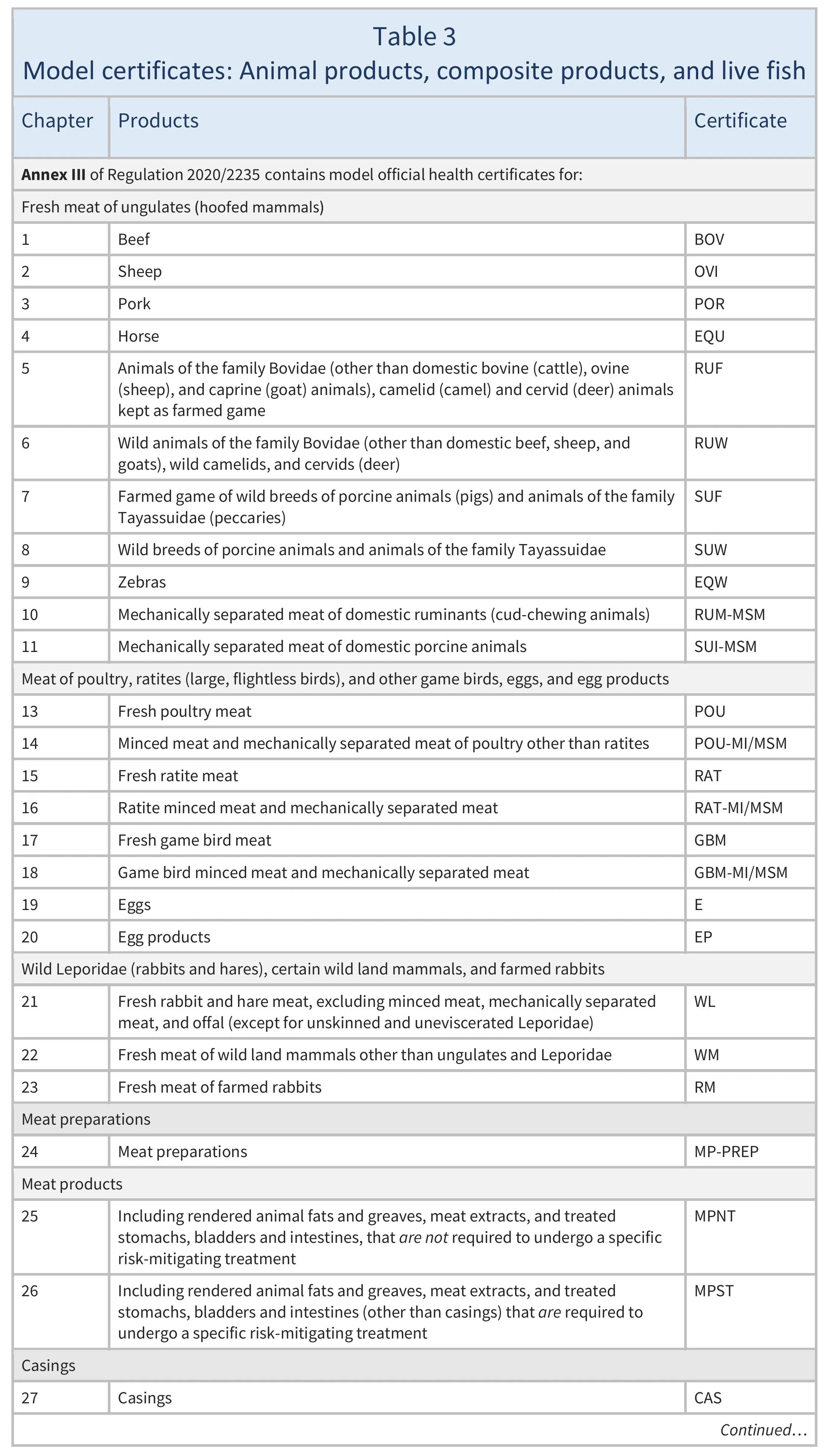
Model certificates
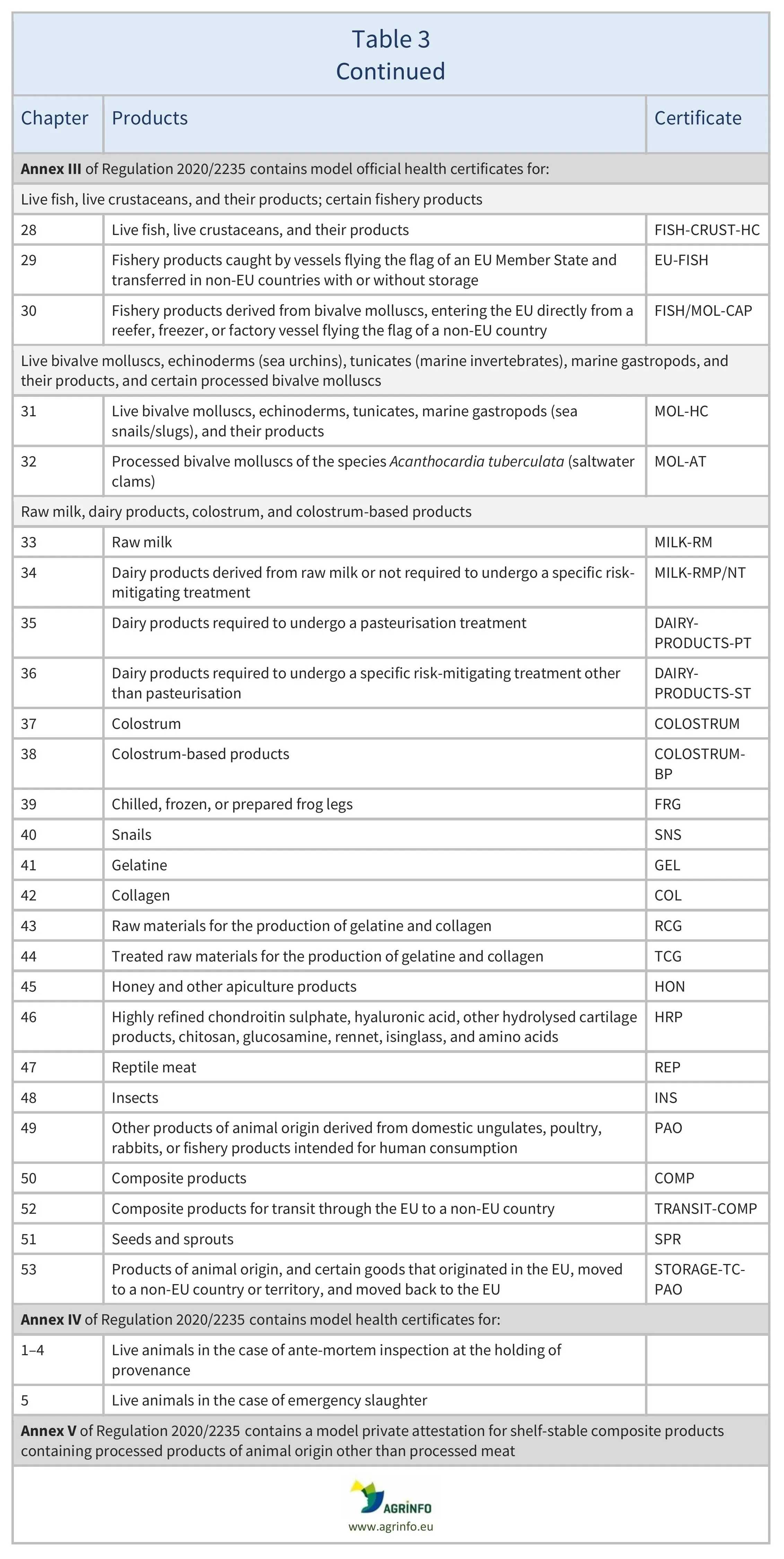
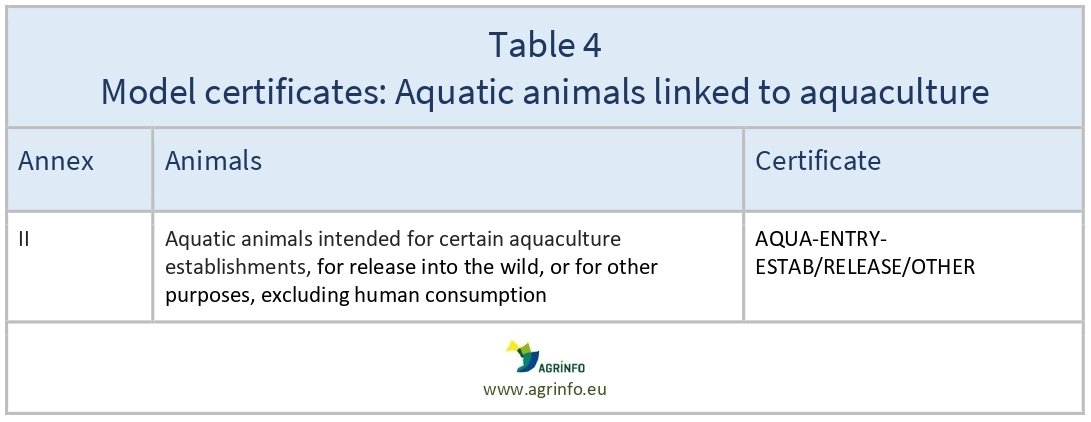
The models of the certificates that must be used to export animals and animal products are set out in different Implementing Regulations, as follows:
- live terrestrial animals and germinal products: Regulation 2021/403 (see Table 2)
- animal products, composite products and live fish: Regulation 2020/2235 (see Table 3)
- aquaculture: Regulation 2020/2236 (see Table 4).
Timeline
Certificates are amended regularly; it is important to use the correct one. Using the digital platform TRACES avoids any mistakes – the certificates available in TRACES are always the most up-to-date ones.
What are the major implications for exporting countries?
Any error in filling in the certificate may result in the rejection of consignments at the EU border. It is essential to pay attention to the information provided in the certificates, and to adapt the certificates whenever the EU Regulations are updated. These changes are highlighted by AGRINFO when they occur.
For animals and animal products, there are about 100 different models. It is crucial to identify the correct one to use.
Background
Legal basis
The basic principles and processes for issuing health certificates can be found in the following EU Regulations.
Under the Official Controls Regulation 2017/625:
- definition and explanation of official certificates (Art. 3(27); Arts. 86–90)
- obligation to show the original official certificate at the border control post (Art. 50)
- management of non-compliant consignments entering the EU (Art. 68)
- pre-export controls of official certificates by non-EU countries (Art. 73)
- controls by European Commission experts of non-EU-country legislation and control systems, as part of audits performed by the Commission (Art. 120)
- recognition of equivalence of measures applied in a non-EU country or its regions (Art. 129)
- actions to take in the event of non-compliance (Art. 138).
Under the Animal Health Law, Regulation 2016/429:
- issuing of animal health certificates issued by the competent authority of the non-EU country (Art. 237)
- the content of animal health certificates (Art. 238).
Resources
Online resources from the European Commission:
- About the Animal Health Law
- Animal health is your health (in 24 languages)
- Video: Animal Health Law (in 24 languages)
- List of delegated and implementing acts (as of 5 September 2022)
- TRACES NT Documentation
Sources
Regulation (EU) 2016/429 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (Animal Health Law)
Commission Implementing Regulations:
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.